Project of Publicly Offered Research
- Home
- Organization
- Project of Publicly Offered Research
A01: Fundamental research on generative brain systems for human individuality
The effect of VMAT1 variants on human personality traits.

-
Project leader
Graduate School of Life Sciences
Professor Masakado Kawata
Further information
Message from Project leader
It has been suggested that the evolution of human specific social behavior and cognitive ability had caused psychiatric disorders. On the other hand, in many cases, these psychiatric disorders-relevant genes show allelic polymorphisms, which could contribute to a human personality. Thus, we focus on psychiatric disorders-relevant genes which have been subject to positive natural selection through evolutionary lineages from nonhuman species and these genes in which allelic polymorphisms are maintained by balancing selection in the present human population. We could understand the mechanisms of the evolution of human mental personality by examining the cause of maintenance of these alleles and the effect of the allelic differences on mental differences.
Our previous study showed that VMAT1 could be detected as a positively selected gene in human lineage. Among them, VMAT1, vesicular monoamine transporter 1, is particularly interesting since it has a human-unique polymorphism (Thr/Ile) in the 136th amino acid site compared to other mammals (Asn). This, in this project, we will try to examine the cause of the maintenance of polymorphism of VMAT1 by using Tohoku Megabank database for genomic and mental condition data. In addition, we will examine the phenotypic effects of this allelic polymorphism by creating genome editing mice.
-
Project leader
ProfessorMasakado KawataGraduate School of Life Sciences
Research Area:
Evolutionary BiologyE-mail:
kawata*tohoku.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Sato, D. X. and M. Kawata (2018) Positive and balancing selection on SLC18A1 gene associated with psychiatric disorders and human-unique personality traits. Evolution Letters 2:499-510
- Sakai, Y., S. Kawamura, and M. Kawata (2018) Genetic and plastic variation in opsin gene expression, light sensitivity, and female response to visual signals in the guppy. Proceedings of the National Academy of Sciences of the United States of America 115:12247-12252
- Akashi, H. D., A. Cadiz, S. Shigenobu, T. Makino and M. Kawata. Differentially expressed genes associated with adaptation to different thermal environments in three sympatric Cuban Anolis lizards. Molecular Ecology, 25, 2273-2285, 2016
- Wallberg, A., F. Han, G. Wellhagen, B. Dahle, M. Kawata, N. Haddad, Z. L. P. Simões,, M. H. Allsopp, I. Kandemir, P. D. la Rúa, C.W. Pirk, and M. T. Webster. A worldwide survey of genome sequence variation provides insight into the evolutionary history of the honeybee Apis mellifera. Nature Genetics 46, 1081–1088, 2014
- Takahashi, Y., K. Kagawa, E. I. Svensson and M. Kawata. Evolution of increased phenotypic diversity enhances population performance by reducing sexual harassment in damselflies. Nature Communications 5, 4468, 2014
- Tamate, S., M. Kawata and T. MakinoContribution of non-ohnologous duplicated genes to high habitat variability in mammals. Molecular Biology and Evolution 31 ,1779-1786, 2014
- Tezuka, A., S. Kasagi, C. van Oosterhout, M. McMullan, W. M. Iwasaki, D. Kasai, M. Yamamichi, H. Innan, S. Kawamura, and M. Kawata. Divergent selection on opsin gene variation in guppy (Poecilia reticulata) populations of Trinidad and Tobago. Heredity 113, 381–389, 2014
- Makino, T., McLysaght, A. and Kawata, M. Genome-wide deserts for copy number variation in vertebrates. Nature Communications 4:2283, 2013
- Makino, T. and Kawata, M. Habitat variability correlates with duplicate content of Drosophilagenomes. Molecular Biology and Evolution, 29, 3169-3179, 2014
- Tsuda, E. M. and M. Kawata (2010) Evolution of gene regulatory networks by fluctuating selection and intrinsic constraints. PLoS Computational Biology 6(8): e1000873, 2010
Close
Development of a novel prediction model of neurodevelopmental outcome in preterm infants based on sulcal pattern analysis

-
Project leader
Department of Pediatrics, Nagoya University Graduate School of Medicine
Assistant Professor Hiroyuki Kidokoro
Further information
Message from Project leader
The aims of our study are first to predict neurodevelopmental outcome by sulcal variations on MRI at term age, and second to clarify the association between sulcal pattern and perinatal variables in children born preterm.
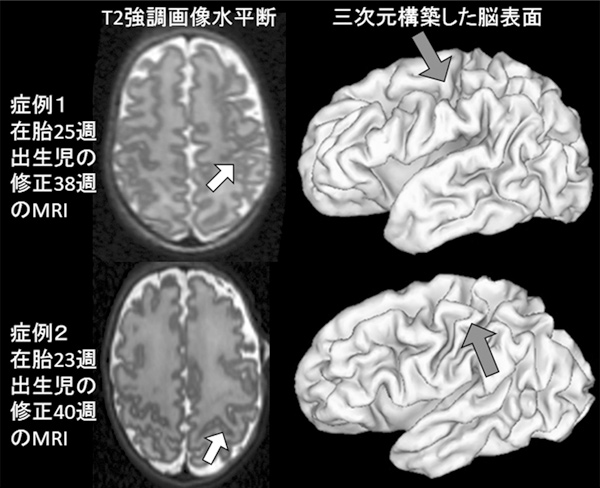
-
Project leader
Assistant ProfessorHiroyuki KidokoroDepartment of Pediatrics, Nagoya University Graduate School of Medicine
Research Area:
Child NeurologyE-mail:
kidokoro*med.nagoya-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Sakaguchi Y, Kidokoro H, Ogawa C, Okai Y, Ito Y, Yamamoto H, Ohno A, Nakata T, Tsuji T, Nakane T, Kawai H, Kato K, Naganawa S, Natsume J. Longitudinal Findings of MRI and PET in West Syndrome with Subtle Focal Cortical Dysplasia.AJNR Am J Neuroradiol,39, 1932-1937, 2018.
- Ogawa C, Kidokoro H, Fukasawa T, Yamamoto H, Ishihara N, Ito Y, Sakaguchi Y, Okai Y, Ohno A, Nakata T, Azuma Y, Hattori A, Kubota T, Tsuji T, Hirakawa A, Kawai H, Natsume J. Cytotoxic edema at onset in West syndrome of unknown etiology: A longitudinal diffusion tensor imaging study.Epilepsia, 59, 440-448, 2018.
- Kidokoro H, de Vries LS, Ogawa C, Ito Y, Ohno A, Groenendaal F, Saitoh S, Okumura A, Ito Y, Natsume J. Predominant area of brain lesions in neonates with herpes simplex encephalitis.J Perinatol. 37, 1210-1214, 2017.
- Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis.Pediatrics,134, e444-53, 2014.
- Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, Reynolds LC, Walker S, Rogers C, Mathur AM, Van Essen DC, Inder T. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments.J Pediatr,164, 52-60.e2, 2014.
- Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term.AJNR Am J Neuroradiol, 34, 2208-14, 2013.
- Kidokoro H, Kubota T, Hayakawa M, Kato Y, Okumura A. Neonatal seizure identification on reduced channel EEG.Arch Dis Child Fetal Neonatal Ed. 98, F359-61, 2013.
- Hayashi-Kurahashi N, Kidokoro H, Kubota T, Maruyama K, Kato Y, Kato T, Natsume J, Hayakawa F, Watanabe K, Okumura A. EEG for predicting early neurodevelopment in preterm infants: an observational cohort study.Pediatrics,130,e891-7,2012.
- Rogers CE, Anderson PJ, Thompson DK, Kidokoro H, Wallendorf M, Treyvaud K, Roberts G, Doyle LW, Neil JJ, Inder TE. Regional cerebral development at term relates to school-age social-emotional development in very preterm children.J Am Acad Child Adolesc Psychiatry,51,181-91,2012.
- Kidokoro H, Okumura A, Hayakawa F, Kato T, Maruyama K, Kubota T, Suzuki M, Natsume J, Watanabe K, Kojima S. Chronologic changes in neonatal EEG findings in periventricular leukomalacia.Pediatrics,124,e468-75,2009.
Close
Realization of the platform of participatory research on autism spectrum disorder based on the social model of disabilities

-
Project leader
Research Center for Advanced Science and Technology, The University of Tokyo
Associate Professor Shin-ichiro Kumagaya
Further information
Message from Project leader
In 1980s, in terms of the epistemology of disability, medical models that bring persons with disabilities closer to the majority was replaced by social models that aim to realize a social environment that includes diverse individualities. In recent years, ASD research based on the viewpoint of users and social model has gradually appeared. Based on the perspective of users and the social model, we have also investigated the individualities that hardly change even if the social environment changes, and clarified the communication style which is easy for ASD to participate in, by the unique approach called “tojisha-kenkyu (user-led study)”. In this project, in order to realize the platform of participatory research on ASD, or “co-production of research” (Nature 2018) of ASD, we will A. establish a training program for tojisha-kenkyu, B. enhance mutual critique between tojisha-kenkyu and academic research, and C. conduct Japanese-UK comparative research on ASD.
-
Project leader
Associate ProfessorShin-ichiro KumagayaResearch Center for Advanced Science and Technology, The University of Tokyo
Research Area:
User led study, PediatricsE-mail:
kumashin*bfp.rcast.u-tokyo.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Ohmura Y, Ichikawa I, Kumagaya S, Kuniyoshi Y: Stapedial reflex threshold predicts individual loudness tolerance for people with autistic spectrum disorders. Experimental Brain Research, 237, 91-109, 2019.
- Lin IF, Hiroya S, Asada K, Ayaya S, Kumagaya S, Kato M: Vocal analysis of speech in adults with autism spectrum disorders. Acoustical Science and Technolog, 39, 154-157, 2018.
- Asada K, Tojo Y, Hakarino K, Saito A, Hasegawa T, Kumagaya, S: Body image in autism: Evidence from body size estimation. Journal of Autism and Developmental Disorders, 48, 611-618, 2018.
- Kumagaya S: Informational support design aiming for the accommodation of individuals with autism spectrum disorders based on the social model of disability: From a user-led research perspective. Journal of the National Institute of Public Health, 66, 532-544, 2017.
- Fukuyama H, Kumagaya S, Asada K, Ayaya S, Kato M: Autonomic versus perceptual accounts for tactile hypersensitivity in autism spectrum disorder. Scientific Reports, 7, 8259, 2017.
- Inui, T, Kumagaya S, Myowa-Yamakoshi M: Neurodevelopmental hypothesis about the etiology of autism spectrum disorders, Frontiers in Human Neuroscience, 11, 354, 2017.
- Osumi H, Sumitani M, Kumagaya S, Morioka S: Optimal control of reaching is disturbed in complex regional pain syndrome (CRPS): a single-case study, Journal of Pain Research, 10, 167-173, 2017.
- Asada K, Tojo Y, Osanai H, Saito A, Hasegawa T, Kumagaya S: Reduced personal space in individuals with autism spectrum disorder. PLoS ONE, 11, e0146306, 2016.
- Lin IF, Mochida T, Asada K, Ayaya S, Kumagaya S, Kato M: Atypical delayed auditory feedback effect and Lombard effect on speech production in high-functioning adults with autism spectrum disorder, Frontiers in Human Neuroscience, 9, 510, 2015.
- Aramaki E, Shikata S, Miyabe M, Usuda Y, Asada K, Ayaya S, Kumagaya S: Understanding the relationship between social cognition and word difficulty. A language based analysis of individuals with autism spectrum disorder. Methods of information in medicine, 54, 522-529, 2015.
- Kumagaya, S: Tojisha-kenkyu of autism spectrum disorders. Advanced Robotics, 29, 25-34, 2015.
- Sumitani M, Misaki M, Kumagaya S, Ogata T, Yamada Y, Miyauchi S: Dissociation in accessing space and number representation in pathologic pain patients. Brain and Cognition, 90, 151-156, 2014.
Close
Individualized brain mapping by deep learning of open resource and out-sample prediction.

-
Project leader
Department of Biosciences and Informatics, Keio University
Associate Professor Koji Jimura
Further information
Message from Project leader
I have been interested in sophisticated human brain mechanisms allowing flexible adaptation to changing environments, and have examined individual differences in the relationships between behavioral characteristics and brain functions. In order to individually predict behavioral profile and elucidate underlying brain mechanisms, the current project aims to develop individualized brain mapping technique. In particular, a predictor of behavioral profile is developed by learning open resource big data of behavioral measures and functional brain images. Then, for out-sample individuals with their data collected independently, the predictor predicts behavioral characteristics and identifies underlying brain mechanisms. As such, the current technique may provide a novel approach to individualize functional mapping of the human brain.
-
Project leader
Associate ProfessorKoji JimuraDepartment of Biosciences and Informatics, Keio University
Research Area:
Cognitive Neuroscience, NeuroinformaticsE-mail:
jimura*bio.keio.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Jimura K, Chushak MS, Westbrook A, Braver TS: Intertemporal decision-making involves prefrontal control mechanisms associated with working memory. Cerebral Cortex, 28, 1105-1116, 2018.
- Jimura K, Hirose S, Kumimatsu K, Ohtomo K, Koike Y, Konishi S: Late enhancement of brain-behavior correlations during response inhibition. Neuroscience, 274, 383-392, 2014.
- Jimura K, Cazalis F, Stover ER, Poldrack RA: The neural basis of task switching changes with skill acquisition. Frontiers in Human Neuroscience, 8, 339, 2014.
- Jimura K, Chushak SM, Braver TS: Impulsivity and self-control during intertemporal decision-making linked to the neural dynamics of reward value representation. Journal of Neuroscience, 33, 344-357, 2013.
- Jimura K, Poldrack RA: Analyses of regional-average activation and multivoxel pattern information tell complementary stories. Neuropsychologia, 50, 544-552, 2012.
- Jimura K, Myerson J, Hilgard J, Keighley J, Braver TS, Green L: Domain independence and stability in young and old adults’ discounting of delayed rewards. Behavioural Processes, 87, 253-259 2011.
- Jimura K, Braver TS: Age-related brain activity dynamics during task switching. Cerebral Cortex, 20, 1420-1431 2010.
- Jimura K, Locke HS, Braver TS: Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of National Academy Sciences of the USA, 107, 8871-8876, 2010.
- Jimura K, Konishi S, Asari T, Miyashita Y: Temporal pole activity during understanding other persons’ mental state correlated with neuroticism trait. Brain Research, 1328, 104-112 2010.
- Jimura K, Myerson J, Hilgard J, Braver TS, Green L: Are people really more patient than other animals? Evidence from human discounting of real liquid rewards. Psychonomic Bulletin and Review, 16, 1071-1075, 2009.
- Jimura K, Konishi S, Miyashita Y: Temporal pole activity during perception of sad faces but not happy faces correlates with neuroticism trait. Neuroscience Letters, 453, 45-48, 2009.
Close
Multilayer informatics analysis on brain and personality focused on microexon

-
Project leader
Division of Interdisciplinary Medical Science, Centers for Advanced Research and Translational Medicine, Graduate School of Medicine, Tohoku University
Lecturer Matsuyuki Shirota
Further information
Message from Project leader
Microexon are about 3-27 nt long short exons and alternative splicing of them results in short insertions or deletions in the amino acid sequence of protein and changes in protein function. Some of the microexons are alternatively spliced in neurons in a developmental-stage specific manner and misregulation of them is reportedly associated with neuropsychiatric diseases, such as autism. However, previous studies did not focus on the effects of genomic variations on splicing of microexons, variations in alternative splicing patterns of microexons among single cells, or the effects of stability of protein structure and protein-protein interactions. By taking advantage of recent genome and transcriptome sequencing data and protein structure data, we perform a multi-layer analysis from genome through transcriptome and protein structure to phenotype focused on microexons and contribute to filling the gap between genome and personality.
-
Project leader
LecturerMatsuyuki ShirotaDivision of Interdisciplinary Medical Science, Centers for Advanced Research and Translational Medicine, Graduate School of Medicine, Tohoku University
Research Area:
Bioinformatics, Protein StructureE-mail:
mshirota*med.tohoku.ac.jp
(Please convert "*" into "@".)URL:
http://www.med.tohoku.ac.jp/english/about/laboratory/230.html
Selected Publication
- Kondo HX, Yoshida N, Shirota M, Kinoshita K. Molecular Mechanism of Depolarization-Dependent Inactivation in W366F Mutant of Kv1.2. J Phys Chem B. 2018 Nov 26. doi: 10.1021/acs.jpcb.8b09446.
- Tadaka S, Saigusa D, Motoike IN, Inoue J, Aoki Y, Shirota M, Koshiba S, Yamamoto M, Kinoshita K. jMorp: Japanese Multi Omics Reference Panel. Nucleic Acids Res. 2018 Jan 4;46(D1):D551-D557. doi: 10.1093/nar/gkx978.
- Shirota M, Kinoshita K. Discrepancies between human DNA, mRNA and protein reference sequences and their relation to single nucleotide variants in the human population. Database (Oxford). 2016 Sep 1;2016. pii: baw124. doi: 10.1093/database/baw124.
- Kasahara K, Shirota M, Kinoshita K. Ion Concentration- and Voltage-Dependent Push and Pull Mechanisms of Potassium Channel Ion Conduction. PLoS One. 2016 Mar 7;11(3):e0150716. doi:10.1371/journal.pone.0150716.
- Shirota M, Kinoshita K. Analyses of the general rule on residue pair frequencies in local amino acid sequences of soluble, ordered proteins. Protein Sci. 2013 Jun;22(6):725-33. doi: 10.1002/pro.2255.
- Kasahara K, Shirota M, Kinoshita K. Comprehensive classification and diversity assessment of atomic contacts in protein-small ligand interactions. J Chem Inf Model. 2013 Jan 28;53(1):241-8. doi: 10.1021/ci300377f.
- Shirota M, Ishida T, Kinoshita K. Absolute quality evaluation of protein model structures using statistical potentials with respect to the native and reference states. Proteins. 2011 May;79(5):1550-63. doi: 10.1002/prot.22982.
- Shirota M, Ishida T, Kinoshita K. Development of a new meta-score for protein structure prediction from seven all-atom distance dependent potentials using support vector regression. Genome Inform. 2009 Oct;23(1):149-58.
- Shirota M, Ishida T, Kinoshita K. Analyses on hydrophobicity and attractiveness of all-atom distance-dependent potentials. Protein Sci. 2009 Sep;18(9):1906-15. doi: 10.1002/pro.201.
- Shirota M, Ishida T, Kinoshita K. Effects of surface-to-volume ratio of proteins on hydrophilic residues: decrease in occurrence and increase in buried fraction. Protein Sci. 2008 Sep;17(9):1596-602. doi: 10.1110/ps.035592.108.
Close
Neural bases of individual differences in cognition: clinical study of epilepsy patients

-
Collaborator
Department of Behavioral Neurology and Cognitive Neuroscience, Tohoku University Graduate School of Medicine
Professor Kyoko Suzuki
Further information
Message from Collaborator
Individual difference in cognitive functions must be underpinned by variation in neural networks of each subject. We aim to find neural bases of individual differences in cognition by electrocortical stimulation mapping and selective Wada testing in patients with intractable epilepsy. These methods are the gold standard to determine intra-hemispheric localization of cognitive functions. The location of eloquent areas must be identified in the individual patient, with the goal of sparing these regions from resection. Epilepsy surgery offers the opportunity for thorough examination prior to resection, enabling within-subject, pre- and postoperative comparisons.
-
Collaborator
ProfessorKyoko SuzukiDepartment of Behavioral Neurology and Cognitive Neuroscience, Tohoku University Graduate School of Medicine
Research Area:
Behavioral Neurology, NeuropsychologyE-mail:
kyon*med.tohoku.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Suzuki K, Yamadori A, Endo K, Fujii T, Ezura M, Takahashi A. Dissociation of letter and picture naming from callosal disconnection. Neurology 51:1390-1394, 1998
- Suzuki K, Otsuka Y, Endo K, Fujii T, Yamadori A. Visuospatial deficits due to impaired visual attention: Investigation of two cases of slowly progressive visuospatial impairment. Cortex 39:327-342, 2003
- Tanji K, Suzuki K, Delorne A, Shamoto H, Nakasato N. High-frequency gamma band activity in the basal temporal cortex during picture naming and lexical decision tasks. Journal of Neuroscience 25:3287-3293, 2005
- Tachibana K, Suzuki K, Mori E, Miura N, Kawashima R, Horie K, Sato S, Tanji J, and Mushiake H. Neural Activity in the Human Brain Signals Logical Rule Identification. Journal of Neurophysiol 102:1526-1537, 2009
- BabaT, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, Hasegawa T, Sugeno N, Konno M, Suzuki K, Takahashi S, Fukuda H, Aoki M, Itoyama Y, Mori E, Takeda A. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3-year longitudinal study. Brain 135:161-169, 2012
- Tanji K, Iwasaki M, Nakasato N, Suzuki K. Face specific broadband electrocorticographic spectral power change in the rhinal cortex. Neuroscience Letter 515:66-70, 2012
- Sawada Y, NIshio Y, Suzuki K, Hirayama K, Takeda A, Hosokai Y, Ishioka T, Itoyama Y, Takahashi S, Fukuda H, Mori E. Attentional set-shifting deficit in Parkinson’s disease is associated with prefrontal dysfunction: an FDG-PET study. PLoS One 7:e38498, 2012
- Tanji K, Sakurada K, Funiu H, Matsuda K, Kayama T, Suzuki K. Functional significance of the electrocorticographic auditory responses in the premotor cortex. Frontiers in Neuroscience 9: 78, Doi:10.3389/fnins.2015.00078, 2015
- Oishi Y, Imamura T, Shimomura T, Suzuki K, Visual texture agnosia in dementia with Lewy bodies and Alzheimer’s disease, Cortex 103, 277-290, 2018
- Ito S, Sato S, Saito N, Ohmuma A, Tobita M, Kimpara T, Iseki C, Suzuki K. Qualitative analysis for the “ideational apraxia” score from the Alzheimer’s Disease Assessment Scale Cognitive Subscale. Neurology and Clinical Neuroscience 2019 (in press)
Close
Molecular mechanism of individual difference of prosociality

-
Project leader
Brain Science Institute, Tamagawa University
Associate Professor Haruto Takagishi
Further information
Message from Project leader
The aim of our laboratory is to elucidate the biological basis of human prosocial behavior. In this project, we examine how the methylation level of the oxytocin receptor gene (OXTR) affects individual differences in prosocial behavior. In addition, we examine what social environmental factors regulate the methylation of the oxytocin receptor gene.
-
Project leader
Associate ProfessorHaruto TakagishiBrain Science Institute, Tamagawa University
Research Area:
Social Neuroscience, Social PsychologyE-mail:
takagishi*lab.tamagawa.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Nishina K, Takagishi H*, Fermin ASR, Inoue-Murayama M, Takahashi H, Sakagami M,Yamagishi T: Association of the oxytocin receptor gene with attitudinal trust: role of amygdala volume. Social cognitive and affective neuroscience, 13, 1091-1097, 2018
- Yamagishi T, Matsumoto Y, Kiyonari T, Takagishi H, Li Y, Kanai R, Sakagami M: Response time in economic games reflects different types of decision conflict for prosocial and proself individuals. Proceedings of National Academy of Sciences U.S.A., 114, 6394-6399, 2017
- Yamagishi, T, Li Y, Fermin ASR, Kanai R, Takagishi H, Matsumoto Y, Kiyonari T, Sakagami M: Behavioural differences and neural substrates of altruistic and spiteful punishment.
Scientific Reports, 7, 14654, 2017 - Fujii T, Schug J, Nishina K, Takahashi T, Okada H, Takagishi H*: Relationship between Salivary Oxytocin Levels and Generosity in Preschoolers. Scientific Reports, 6, 38662, 2016
- Takagishi H*, Fujii T, Nishina K, Okada H: Fear of Negative Evaluation Moderates the Effect of Subliminal Fear Priming on Rejection of Unfair Offers in the Ultimatum Game. Scientific Reports, 6, 31446, 2016
- Yamagishi T, Takagishi H, Fermin ASR, Kanai R, Li Y, Matsumoto Y: Cortical thickness of the dorsolateral prefrontal cortex predicts strategic choices in economic games.
Proceedings of National Academy of Sciences U.S.A., 113, 5582-5587, 2016 - Nishina K, Takagishi H, Inoue-Murayama M, Takahashi H, Yamagishi T: Polymorphism of the Oxytocin Receptor Gene Modulates Behavioral and Attitudinal Trust among Men but Not Women.
PLoS ONE, 10, e0137089, 2015 - Takagishi H*, Fujii T, Koizumi M, Schug J, Nakamura F, Kameshima S: The development of the effect of peer monitoring on generosity differs among elementary school-age boys and girls. Frontiers in Psychology, 6, 895, 2015
- Fujii T, Takagishi H*, Koizumi M, Okada H: The Effect of Direct and Indirect Monitoring on Generosity Among Preschoolers. Scientific Reports, 5, 9025, 2015.
- Yamagishi T, Li Y, Takagishi H, Matsumoto Y, Kiyonari T: In search of homo economicus.
Psychological Science, 25, 1699-1711, 2014.
Close
Understanding the molecular mechanisms behind the inter-individual variability of response to antipsychotics using induced pluripotent stem cell-related technologies

-
Project leader
Department of Bioscience Tokyo University of Agriculture
Professor Takanobu Nakazawa
Further information
Message from Project leader
Schizophrenia is a commonly occurring psychiatric disorder characterized by positive symptoms, negative symptoms and cognitive dysfunction. Currently, much remains unknown about the details of the molecular and cellular etiology of this disorder. Furthermore, given that many patients still fail to achieve sufficient remission after current drug medications, including clozapine treatment, there is a need for new drugs based on new molecular mechanisms. To achieve this, it is important to identify the molecular mechanisms behind the inter-individual variability of response to antipsychotics. In this project, we employ the induced pluripotent stem cell-related technologies to identify the molecular mechanisms behind the inter-individual variability of response to clozapine as well as individual differences of neural functions.
-
Project leader
ProfessorTakanobu NakazawaDepartment of Bioscience Tokyo University of Agriculture
Research Area:
Neuroscience, Molecular and Cellular biologyE-mail:
takanobunakazawa-tky*umin.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Nakazawa T, Kikuchi M, Ishikawa M, Yamamori H, Nagayasu K, Matsumoto T, Fujimoto M, Yasuda Y, Fujiwara M, Okada S, Matsumura K, Kasai A, Hayata-Takano A, Shintani N, Numata S, Takuma K, Akamatsu W, Okano H, Nakaya A, Hashimoto H, Hashimoto R: Differential gene expression profiles in neurons generated from lymphoblastoid B-cell line-derived iPS cells from monozygotic twin cases with treatment-resistant schizophrenia and discordant responses to clozapine. Schizophrenia Research, 181, 75-82, 2017.
- Nakazawa T*, Hashimoto R, Sakoori K, Sugaya Y, Tanimura A, Hashimotodani Y, Ohi K, Yamamori H, Yasuda Y, Umeda-Yano S, Kiyama Y, Konno K, Inoue T, Yokoyama K, Inoue T, Numata S, Ohnuma T, Iwata N, Ozaki N, Hashimoto H, Watanabe M, Manabe T, Yamamoto T, Takeda M, Kano M: Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders. Nature Communications, 7, 10594, 2016 (* corresponding author).
- Hashimoto R, Nakazawa T*, Tsurusaki Y, Yasuda Y, Nagayasu K, Matsumura K, Kawasaki H, Yamamori H, Fujimoto M, Ohi K, Umeda-Yano S, Fukunaga M, Fujino H, Kasai A, Hayata-Takano A, Shintani N, Takeda M, Matsumoto N, Hashimoto H: Whole-exome sequencing and neurite outgrowth analysis in autism spectrum disorder. Journal of Human Genetics, 61, 199-206, 2016 (* corresponding author).
- Matsumura K, Nakazawa T*, Nagayasu K, Gotoda-Nishimura N, Kasai A, Hayata-Takano A, Shintani N, Yamamori H, Yasuda Y, Hashimoto R, Hashimoto H: De novo POGZ mutations in sporadic autism disrupt the DNA-binding activity of POGZ. Journal of Molecular Psychiatry, 4, 1, 2016 (* corresponding author).
- Delawary M, Tezuka T, Kiyama Y, Yokoyama K, Inoue T, Hattori S, Hashimoto R, Umemori H, Manabe T, Yamamoto T, Nakazawa T*: NMDAR2B tyrosine phosphorylation regulates anxiety-like behavior and CRF expression in the amygdala. Molecular Brain, 3, 37, 2010 (* corresponding author).
- Nakazawa T*, Taniguchi S, Tanimura A, Kiyama Y, Tezuka T, Watabe AM, Katayama N, Yokoyama K, Inoue T, Izumi-Nakaseko H, Kakuta S, Sudo K, Iwakura Y, Umemori H, Inoue T, Murphy NP, Hashimoto K, Kano M, Manabe T, Yamamoto, T: Involvement of NMDAR2A tyrosine phosphorylation in depression-related behaviour. The EMBO Journal, 28, 3717-3729, 2009 (* corresponding author).
- Nakazawa T, Kuriu T, Tezuka T, Umemori H, Okabe S, Yamamoto T: Regulation of dendritic spine morphology by an NMDA receptor-associated RhoGTPase-activating protein, p250GAP. Journal of Neurochemistry, 105, 1384-1393, 2008.
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T: NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. The EMBO Journal, 25, 2867-2877, 2006.
- Nakazawa T, Watabe AM, Tezuka T, Yoshida Y, Yokoyama K, Umemori H, Inoue A, Okabe S, Manabe T, Yamamoto T: p250GAP, a novel brain-enriched GTPase-activating protein for Rho family GTPases, is involved in the N-methyl-D-aspartate receptor signaling. Molecular Biology of the Cell, 14, 2921-2934, 2003.
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T: Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. Journal of Biological Chemistry, 276, 693-699, 2001.
Close
Establishing animal personality measures for cross-species personality research

-
Project leader
Wildlife Research center, Kyoto University
Professor Miho Murayama
Further information
Message from Project leader
The purpose of this study is to establish reliable measures for animal personality and compare them across species. In several animal species we plan to identify fundamental dimensions of individual differences in behavior based on data such as behavior observations and ratings. We will also apply the same methods of measuring personality to humans. This will enable us to extract dimensions common to personality differences in humans and animals. By obtaining molecular markers that may be related to personality, i.e., genotypes and hormone levels, we will pursue three topics: 1) the comparison of different ways of assessing personality, 2) the comparison of the relationships between personality and the markers, and 3) identifying a common model of personality across species.
-
Project leader
ProfessorMiho MurayamaWildlife Research center, Kyoto University
Research Area:
Genetics, Animal BehaviorE-mail:
mmurayama*wrc.kyoto-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Inoue-Murayama M: Genetic polymorphism as a background of animal behavior (review). Animal Science Journal, 80: 113-120, 2009.
- Hong KW, Weiss A, Morimura N, Udono T, Hayasaka I, Humle T, Murayama Y, Ito S, Inoue-Murayama M: Polymorphism of the tryptophan hydroxylase 2 (TPH2) gene is associated with chimpanzee neuroticism. PLoS One, 6:e22144, 2011.
- Yasui S, Konno A, Tanaka M, Idani G, Ludwig A, Lieckfeldt D, Inoue-Murayama M: Personality assessment and its association with genetic factors in captive Asian and African elephants. Zoo Biology, 32(1):70-78, 2013.
- Abe H, Aoya D, Takeuchi H, Inoue-Murayama M: Gene expression patterns of chicken. Neuregulin 3 in association with copy number variation and frameshift deletion. BMC Genetics, 18(1): 69, 2017.
- Hori Y, Tozaki T, Nambo Y, Sato F, Ishimaru M, Inoue-Murayama M, Fujita K: Evidence for effect of serotonin receptor 1A gene (HTR1A) polymorphism on tractability in Thoroughbred horses. Animal Genetics, 47(1):62-7, 2015.
- Arahori M, Inoue-Murayama M, Fujita K: Cat breed differences in microsatellites adjacent to oxytocin receptor gene (OXTR) and vasopressin receptor gene (AVPR1A). Frontiers in Psychology, 8: 2165, 2017.
- Wilson V, Weiss A, Humle T, Morimura N, Udono T, Idani G, Matsuzawa T, Hirata S, Inoue-Murayama M: Chimpanzee Personality and the Arginine Vasopressin Receptor 1A Genotype. Behavior Genetics, 47(2): 215-226, 2017.
- Ramadan S, Nowier AM, Hori Y, Inoue-Murayama M: The association between glutamine repeats in the androgen receptor gene and personality traits in dromedary camel (Camelus dromedarius). PLoS One, 13(2):e0191119, 2018.
- Konno A, Inoue-Murayama M, Yabuta S, Tonoike A, Nagasawa M, Mogi K, Kikusui T: Effect of Canine Oxytocin Receptor Gene Polymorphism on the Successful Training of Drug Detection Dogs, Journal of Heredity, 109(5):566-572, 2018.
- Inoue-Murayama M, Yokoyama C, Yamanashi Y, Weiss A: Common marmoset (Callithrix jacchus) personality, subjective well-being, hair cortisol level and AVPR1a, OPRM1, and DAT genotypes. Scientific Reports, 8(1):10255, 2018.
Close
Individuality of embodiment by individual differences of sensory processing

-
Project leader
Department of Rehabilitation for Brain Functions, Research Institute of National Rehabilitation Center for Persons with Disabilities
Section Chief of Developmental Disorders Section Makoto Wada
Further information
Message from Project leader
In this study, we will elucidate neuro-cognitive basis of individuality of the embodiment, which is linked to be each speciality and inaptness in daily life. From previous our studies, touch perception could not be allocated outside the body in part of individuals with autism spectrum disorder (ASD), and the embodiment of tools tend to be difficult in them. Thus, cognitive style of the embodiment, as a kind of individuality, might be linked to speciality and inaptness about tool use or ball games in daily life. However, it doesn’t mean simple categories such as ASD and the others, because we have observed large individual differences in various experiments. That is, neurodiversity related to sensorimotor processing about the body seems to produce individuality of the embodiment. In this study, we will investigate the sensorimotor systems that are connected to the individuality of the embodiment by using psychophysics, neuroimaging and interviews.
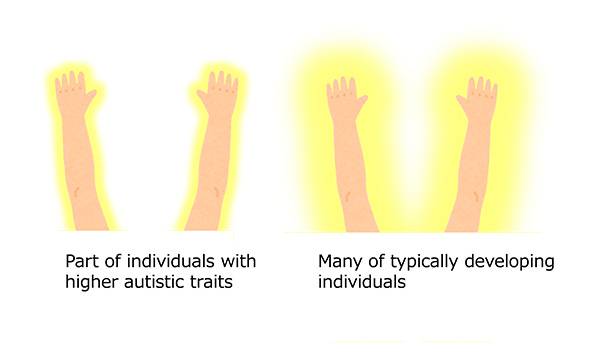
-
Project leader
Section Chief of Developmental Disorders SectionMakoto WadaDepartment of Rehabilitation for Brain Functions, Research Institute of National Rehabilitation Center for Persons with Disabilities
Research Area:
Cognitive science, NeurophysiologyE-mail:
wada-makoto*rehab.go.jp
(Please convert "*" into "@".)URL:
http://www.rehab.go.jp/ri/noukinou/hattatsu/index_eng.html
Selected Publication
- Hidaka S, Suzuishi Y, Ide M, Wada M. Effects of spatial consistency and individual difference on touch-induced visual suppression effect. Scientific Reports. 8, 17018, 2018.
- Fukui T, Sano M, Tanaka A, Suzuki M, Kim S, Agarie H, Fukatsu R, Nishimaki K, Nakajima Y, Wada M. Older adolescents and young adults with autism spectrum disorder have difficulty chaining motor acts when performing prehension movements compared to typically developing peers. Frontiers in Human Neuroscience, 12: 430. 2018.
- Atsumi T, Ide M, Wada M. Spontaneous discriminative response to the biological motion displays involving a walking conspecific in mice. Frontiers in Behavioral Neuroscience. 12, 263. 2018.
- Ide, M., Yaguchi, A., Sano, M., Fukatsu, R., & Wada, M. Higher tactile temporal resolution as a basis of hypersensitivity in individuals with autism spectrum disorder. Journal of Autism and Delopmental Disorders, 49(1)44-53, 2019
- Ide M & Wada M. Salivary oxytocin concentration associates with the subjective feeling of body ownership during the rubber hand illusion. Frontiers in Human Neuroscience, 11:166, doi:10.3389/fnhum.2017.00166, 2017.
- Wada M, Takano K, Ora H, Ide M & Kansaku K. The Rubber Tail Illusion as Evidence of Body Ownership in Mice. Journal of Neuroscience, 36(43):11133-11137, 2016.
- Ide M, Hidaka S, Ikeda H & Wada M. Neural mechanisms underlying touch-induced visual perceptual suppression: An fMRI study. Scientific Reports, 6, 37301, 2016.
- Wada M & Ide M. Rubber hand presentation modulates visuotactile interference effect, especially in persons with high autistic traits. Experimental Brain Research, 234(1):51-65, 2016.
- Nishikawa N, Shimo Y, Wada M, Hattori N & Kitazawa S. Effects of aging and idiopathic Parkinson’s disease on tactile temporal order judgment. PLoS One. 10(3) e0118331, 2015.
- Wada M, Suzuki M, Takaki A, Miyao M, Spence C & Kansaku K. Spatio-temporal processing of tactile stimuli in autistic children. Scientific Reports, 4:5985, 2014.
Close
A02: Fundamental research on generative brain systems for animal individuality
Analysis of genetic and environmental factors to define individuality of spatial pattern separation

-
Project leader
Graduate School of Science, Nagoya University
Lecturer Natsumi Ageta-Ishihara
Further information
Message from Project leader
It is commonly accepted that genetic and environmental factors establish “individuality.” However, there are a few tools and technologies available to elucidate individuality. We will establish molecular and neural bases to define individuality of spatial pattern separation using model mice and evaluate environmental factors to influence pattern separation.
-
Project leader
LecturerNatsumi Ageta-IshiharaGraduate School of Science, Nagoya University
Research Area:
Neuroscience, NeurochemistryE-mail:
ageta-ishihara.natsumi*h.mbox.nagoya-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Ageta-Ishihara N, Konno K, Yamazaki M, Abe M, Sakimura K, Watanabe M, Kinoshita M: CDC42EP4, a perisynaptic scaffold protein in Bergmann glia, is required for glutamatergic tripartite synapse configuration. Neurochemistry International, 119, 190-198, 2018
- Parajuli L, Ageta-Ishihara N, Ageta H, Fukazawa Y, Kinoshita M: Methods for Immunoblot Detection and High-resolution Subcellular Mapping of Septin. Methods in Cell Biology, Septins, 136, Chapter 16, 2016
- Horigane S*, Ageta-Ishihara N*(*equal contribution), Kamijo S, Fujii H, Okamura M, Kinoshita M, Takemoto-Kimura S, Bito H: Facilitation of axon outgrowth via a Wnt5a-CaMKK-CaMKIα pathway during neuronal polarization. Molecular brain, 9(1), 8, 2016
- Ageta-Ishihara N, Yamazaki M, Konno K, Nakayama H, Abe M, Hashimoto K, Nishioka T, Kaibuchi K, Hattori S, Miyakawa T, Tanaka K, Huda F, Hirai H, Hashimoto K, Watanabe M, Sakimura K, Kinoshita M: A CDC42EP4/septin-based perisynaptic glial scaffold facilitates glutamate clearance. Nature Communications, 6, 10090, 2015
- Ageta-Ishihara N, Miyata T, Ohshima C, Watanabe M, Sato Y, Hamamura Y, Higashiyama T, Mazitschek R, Bito H, Kinoshita M: Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nature Communications, 4, 2532, 2013
- Ageta-Ishihara N, Yamakado H, Morita T, Hattori S, Takao K, Miyakawa T, Takahashi R, Kinoshita M: Chronic overload of SEPT4, a parkin substrate that aggregates in Parkinson's disease, causes behavioral alterations but not neurodegeneration in mice. Molecular brain, 6, 35, 2013
- Ageta-Ishihara N, Takemoto-Kimura S, Nonaka M, Adachi-Morishima A, Suzuki K, Kamijo S, Fujii H, Mano T, Blaeser F, Chatila TA, Mizuno H, Hirano T, Tagawa Y, Okuno H, Bito H: Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade. Journal of Neuroscience, 29, 13720-13729, 2009
- Takemoto-Kimura S*, Ageta-Ishihara N*(*equal contribution), Nonaka M, Adachi-Morishima A, Mano T, Okamura M, Fujii H, Fuse T, Hoshino M, Suzuki S, Kojima M, Mishina M, Okuno H, Bito H: Regulation of dendritogenesis via a lipid raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIgamma. Neuron, 54, 755-770, 2007
Close
Molecular and neural mechanisms of individual differences in behavioral responses to chronic stress

-
Project leader
Medical Innovation Center, Kyoto University Graduate School of Medicine
Research Associate Professor Shusaku Uchida
Further information
Message from Project leader
Stressful events during adulthood are potent adverse environmental factors that can predispose individuals to psychiatric disorders, including depression; however, many individuals exposed to stressful events can adapt and function normally. Thus, individuals exposed to chronic stress display wide-ranging, heterogeneous responses. While stress susceptibility may influence depression, the molecular mechanisms underlying the susceptibility and resilience to chronic stress within the brain remain to be elucidated. In this study, we focus on the epigenetic regulation of gene transcription as a key mechanism underlying individual differences in stress susceptibility.
-
Project leader
Research Associate ProfessorShusaku UchidaMedical Innovation Center, Kyoto University Graduate School of Medicine
Research Area:
Neuroscience, Molecular biologyE-mail:
uchida.shusaku.3n*kyoto-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Uchida S, Yamagata H, Seki T, Watanabe Y. Epigenetic mechanisms of major depression: Targeting neuronal plasticity. Psychiatry Clin Neurosci 72, 212-227, 2018
- Uchida S, Teubner BJ, Hevi C, Hara K, Kobayashi A, Dave RM, Shintaku T, Jaikhan P, Yamagata H, Suzuki T, Watanabe Y, Zakharenko SS, Shumyatsky GP. CRTC1 Nuclear Translocation Following Learning Modulates Memory Strength via Exchange of Chromatin Remodeling Complexes on the Fgf1 Gene. Cell Reports 18, 352-366, 2017
- Abe-Higuchi N, Uchida S, Yamagata H, Higuchi F, Hobara T, Hara K, Kobayashi A, Watanabe Y: Hippocampal Sirtuin 1 Signaling Mediates Depression-like Behavior. Biological Psychiatry 80, 808-809, 2016
- Higuchi F, Uchida S, Yamagata H, Abe-Higuchi N, Hobara T, Hara K, Kobayashi A, Shintaku T, Itoh Y, Suzuki T, Watanabe Y: Hippocampal MicroRNA-124 Enhances Chronic Stress Resilience in Mice. The Journal of Neuroscience 36, 7253-7267, 2016
- Uchida S, Martel G, Pavlowsky A, Takizawa S, Hevi C, Watanabe Y, Kandel ER, Alarcon JM, Shumyatsky GP: Learning-induced and stathmin-dependent changes in microtubule stability are critical for memory and disrupted in ageing. Nature Communications 5, 4389, 2014
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y: Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron 69, 359-372, 2011
- Uchida S, Hara K, Kobayashi A, Fujimoto M, Otsuki K, Yamagata H, Hobara T, Abe N, Higuchi F, Shibata T, Hasegawa S, Kida S, Nakai A, Watanabe Y: Impaired hippocampal spinogenesis and neurogenesis and altered affective behavior in mice lacking heat shock factor 1. PNAS 108, 1681-1686, 2011
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y: Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. The Journal of Neuroscience 30, 15007-15018, 2010
Close
Projection of brain individuality by cell ensemble activities representing cognitive information

-
Project leader
Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama
Lecturer Noriaki Ohkawa
Further information
Message from Project leader
From our current work, it is suggested that whole image of an episodic memory is represented as orchestrated activities of cell ensembles in brain. However, it remains unclear whether the ensemble representation is specific to each individual subject. To address brain individuality of cognitive function, we will propose to project preference of cognitive information in each subject by cell ensemble activities during episodic memory processing.
-
Project leader
LecturerNoriaki OhkawaGraduate School of Medicine and Pharmaceutical Sciences, University of Toyama
Research Area:
Neuroscience, Molecular biologyE-mail:
nohkawa*med.u-toyama.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Ghandour K #, Ohkawa N #,*, Fung C. C. A #, Asai H, Saitoh Y, Takekawa T, Okubo-Suzuki R, Soya S, Nishizono H, Matsuo M, Sato M, Ohkura M, Nakai J, Hayashi Y, Sakurai T, Osanai M, Kitamura T, Fukai T, Inokuchi K * (# Equally contributed, * Corresponding authors): Orchestrated ensemble activities constitute a hippocampal memory engram.
Nature Communications, 10, 2637, 2019 - Đặng TC, Ishii Y, Nguyen V, Yamamoto S, Hamashima T, Okuno N, Nguyen QL, Sang Y, Ohkawa N, Saitoh Y, Shehata M, Takakura N, Fujimori T, Inokuchi K, Mori H, Andrae J, Betsholtz C, Sasahara M: Powerful Homeostatic Control of Oligodendroglial Lineage by PDGFRα in Adult Brain.
Cell Reports, 27, 1073-1089, 2019 - Oishi N, Nomoto M, Ohkawa N, Saitoh Y, Sano Y, Tsujimura S, Nishizono H, Matsuo M, Muramatsu S, Inokuchi K: Artificial association of memory events by optogenetic stimulation of hippocampal CA3 cell ensembles.
Molecular Brain, 12, 2, 2019 - Yokose J, Okubo-Suzuki R, Nomoto M, Ohkawa N, Nishizono H, Suzuki A, Matsuo M, Tsujimura S, Takahashi Y, Nagase M, Watabe AM, Sasahara M, Kato F, Inokuchi K: Overlapping memory trace indispensable for linking, but not recalling, individual memories.
Science, 355, 398-403, 2017 - Nomoto M, Ohkawa N, Nishizono H, Yokose J, Suzuki A, Matsuo M, Tsujimura S, Takahashi Y, Nagase M, Watabe AM, Kato F, Inokuchi K: Cellular tagging as a neural network mechanism for behavioral tagging.
Nature Communications, 7, 12319, 2016 - Ohkawa N, Saitoh Y, Suzuki A, Tsujimura S, Murayama E, Kosugi S, Nishizono H, Matsuo M, Takahashi Y, Nagase M, Sugimura YK, Watabe AM, Kato F, Inokuchi K: Artificial association of pre-stored information to generate a qualitatively new memory.
Cell Reports, 11, 261-269, 2015
Close
The social and genetic mechanisms of behavioral variation in ants and its adaptive significance.

-
Project leader
Department of Biological Sciences, Tokyo Metropolitan University
Associate Professor Yasukazu Okada
Further information
Message from Project leader
Social interactions such as parent-child interaction and sexual interaction, have fundamental effects on the development of animal personality. As in group-living vertebrates, social insects such as ants and bees often develop behavioral variation based on social interactions. Interestingly, such behavioral variation is considered to be functional in colony-level activities. We focus on the behavioral variability of ants in this project. First, we quantify social interaction and behavioral variability among nest-mates using the automated behavioral tracking. Second, molecular basis of behavioral variation is analyzed by examining the brain gene expression patterns. We will try to integrate the ultimate and proximate factors and that generate the personality and social organization.

-
Project leader
Associate ProfessorYasukazu OkadaDepartment of Biological Sciences, Tokyo Metropolitan University
Research Area:
Ecologial developmental biology, ethologyE-mail:
okayasukazu*gmail.com
(Please convert "*" into "@".)
Selected Publication
- Ant activity-rest rhythms vary with age and interaction frequencies of workers.
Fujioka H, Abe MS, Okada Y*.
Behavioral Ecology and Sociobiology (2019) 73.3: 30. - Social dominance alters nutrition-related gene expression immediately: transcriptomic evidence from a monomorphic queenless ant.
Okada Y*, Watanabe Y, Mandy MYT, Tsuji K, Mikheyev AS*.
Molecular Ecology (2017) 26: 2922-2938. - Ant circadian activity associated with brood care type
Fujioka H, Abe MS, Fuchikawa T, Tsuji K, Shimada M, Okada Y*.
Biology Letters (2017) 13: 20160743. - Queen contact and among-worker interactions dually suppress worker brain dopamine as a potential regulator of reproduction in an ant.
Shimoji H*, Aonuma H, Miura T, Tsuji K, Sasaki K, Okada Y*.
Behavioral Ecology and Sociobiology (2017) 71: 35. - Histone deacetylases control module-specific phenotypic plasticity in beetle weapons.
Ozawa T, Mizuhara T, Arata M, Shimada M, Niimi T, Okada K, Okada Y*, Ohta K*.
Proceedings of the National Academy of Sciences (2016) 113: 15042-15047. - Mutual intra- and interspecific social parasitism between parapatric sister species of Vespula wasps.
Saga T*, Kanai M, Shimada M, Okada Y.
Insectes Sociaux (2017) 64: 95 - Social dominance and reproductive differentiation mediated by the
dopaminergic signaling in a queenless ant.
Okada Y*, Sasaki K, Miyazaki S, Shimoji H, Tsuji K, Miura T.
Journal of Experimental Biology (2015) 218, 1091-1098 - Morphological variability of intercastes in the ant Temnothorax nylanderi: pattern of trait expression and modularity.
Okada Y, Plateaux L, Peeters C.
Insectes Sociaux (2013) 60: 319-328 - Ovarian development and insulin-signaling pathways during reproductive differentiation in the queenless ponerine ant Diacamma sp.
Okada Y., Miyazaki S., Miyakawa H., Ishikawa A., Tsuji K., Miura T
Journal of Insect Physiology (2010) 56, 288-295
Close
How to modulate individuality

-
Project leader
Graduate School of Frontier Biosciences, Osaka University
Associate Professor Ryosuke Kaneko
Further information
Message from Project leader
Individuality is created by molecules that are responsible for formation of specific neural circuits. We are focusing on clustered protocadherins (Pcdh) as a candidate for individuality creator. In this study, we are addressing following questions using fluorescent protein knock-in mice: (a) Quantification of Pcdh dynamism, (b) Identifying underlying mechanism of Pcdh expression, (c) Analyzing relationship between Pcdh dynamism and the individuality modulation. These experiments will answer how to modulate individuality.
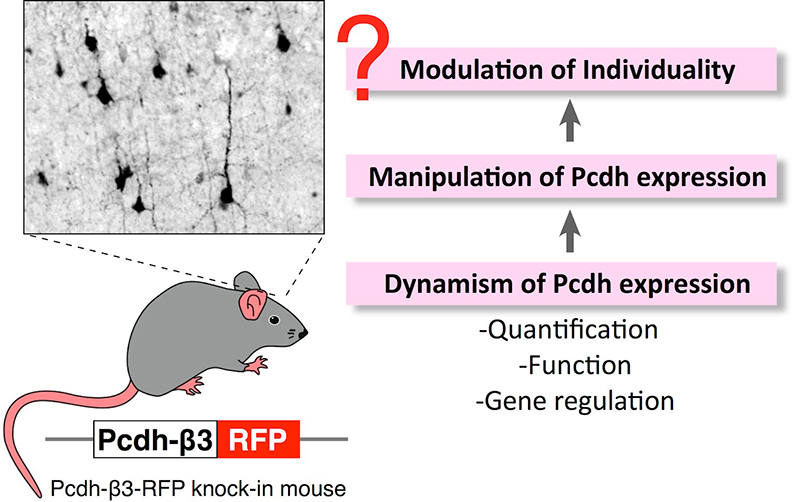
-
Project leader
Associate ProfessorRyosuke KanekoGraduate School of Frontier Biosciences, Osaka University
Research Area:
Neurobiology, Laboratory Animal ScienceE-mail:
rkaneko*fbs.osaka-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Kaneko R, Kakinuma T, Sato S, Jinno-Oue A: Freezing sperm in short straws reduces storage space and allows transport in dry ice. J Reprod Dev. 64, 541-545, 2018
- Kaneko R, Takatsuru Y, Morita A, Amano I, Haijima A, Imayoshi I, Tamamaki N, Koibuchi N, Watanabe M, Yanagawa Y: Inhibitory neuron-specific Cre-dependent red fluorescent labeling using VGAT BAC-based transgenic mouse lines with identified transgene integration site. Journal of Comparative Neurology, 526, 373-396, 2018
- Tarusawa E, Sanbo M, Okayama A, Miyashita T, Kitsukawa T, Hirayama T, Hirabayashi T, Hasegawa S, Kaneko R, Toyoda S, Kobayashi T, Kato-Itoh M, Nakauchi H, Hirabayashi M, Yagi T, Yoshimura Y: Establishment of high reciprocal connectivity between clonal cortical neurons is regulated by the Dnmt3b DNA methyltransferase and clustered protocadherins. BMC Biology, 14, 103, 2016
- Kaneko R, Abe M, Hirabayashi T, Uchimura A, Sakimura K, Yanagawa Y, Yagi T: Expansion of stochastic expression repertoire by tandem duplication in mouse Protocadherin-a cluster. Scientific Reports 4, 6263, 2014
- Kaneko R, Kakinuma T, Sato A, Jinno-Oue A, Hata H: Littermate influence on infant growth in mice: Comparison of SJL/J and ICR as cotransferred carrier embryos. Experimental Animals, 63, 375-381, 2014
- Hirano K, Kaneko R (co-first author), Izawa T, Kawaguchi M, Kitsukawa T, Yagi T: Single-neuron diversity generated by Protocadherin-β cluster in mouse central and peripheral nervous systems. Front Mol Neurosci. 5, 90, 2012
- Shino M, Kaneko R (co-first author), Yanagawa Y, Kawaguchi Y, Saito Y: Electrophysiological characteristics of inhibitory neurons of the prepositus hypoglossi nucleus as analyzed in Venus-expressing transgenic rats. Neuroscience, 197, 89-98, 2011
- Kaneko R, Kawaguchi M, Toyama T, Taguchi T, Yagi T: Expression levels of Protocadherin-a transcripts are decreased by nonsense-mediated mRNA decay with frameshift mutations and by high DNA methylation in their promoter regions. Gene, 430, 86-94, 2009
- Esumi S, Kaneko R, Kawamura Y, Yagi T: Split single cell RT-PCR analysis of Purkinje cells. Nature Protocols, 1, 2143-2151, 2006
- Kaneko R, Kato H, Kawamura Y, Esumi S, Hirayama T, Hirabayashi T, Yagi T: Allelic gene regulation of Pcdh-a and Pcdh-g clusters involving both monoallelic and biallelic expression in single Purkinje cells. J. Biol. Chem., 281, 30551-30560, 2006
Close
Reproductive and neuroendocrinological properties corresponding to individual differences in courtship ultrasonic vocalizations of male mice

-
Project leader
Lab. of Neuroscience, Course of Psychology, Department of Humanities, Faculty of Law, Economics and the Humanities, Kagoshima University
Associate Professor Kouta Kanno
Further information
Message from Project leader
Courtship ultrasonic vocalizations (USVs) of male mice have been well documented and are thought to have an important role in male-female communication, functioning as a type of sexual behavior known as precopulatory behavior. Since the recent discovery of their song-like structure, USVs have attracted numerous research interest (Holy and Guo, PLOS Biol, 2005). USVs have often been used to study biolinguistics, social behaviors, vocal communication, and disorders that involve abnormalities in social behavior, especially autism spectrum disorders (ASDs).
Because singing in some songbirds is acquired by vocal learning, it was hypothesized that a similar learning process may occur in mice; however, several studies revealed that vocalizations in mice are mainly determined genetically. Mice use specific call types depending on their genetic strain. However, we observed that the number of calls and types of call repertories differ among individual mice, even among mice from the same inbred strain, such as C57BL/6. We are now focusing on the individuality observed in mouse vocalizations and the neural and hormonal mechanisms underlying this process.
-
Project leader
Associate ProfessorKouta KannoLab. of Neuroscience, Course of Psychology, Department of Humanities, Faculty of Law, Economics and the Humanities, Kagoshima University
Research Area:
Ultrasonic vocalizations in mice, Behavioral neuroscienceE-mail:
canno*leh.kagoshima-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Tachibana RO, Kanno K, Okabe S, Kobayasi KI, Okanoya K: USVSEG: A robust segmentation of rodents’ ultrasonic vocalization. bioRxiv [11 Mar 2019] doi:10.1101/572743. PPR:PPR72732.
- Kanno K, Kikusui T: Effect of Sociosexual Experience and Aging on Number of Courtship Ultrasonic Vocalizations in Male Mice. Zoolog Sci. 2018 Jun;35(3) 208-214. doi:10.2108/zs170175.
- Yasumura M, Yoshida T, Yamazaki M, Abe M, Natsume R, Kanno K, Uemura T, Takao K, Sakimura K, Kikusui T, Miyakawa T, Mishina M: IL1RAPL1 knockout mice show spine density decrease, learning deficiency, hyperactivity and reduced anxiety-like behaviours. Sci Rep. 2014;4 6613. doi:10.1038/srep06613.
- Hattori T, Kanno K, Nagasawa M, Nishimori K, Mogi K, Kikusui T: Impairment of interstrain social recognition during territorial aggressive behavior in oxytocin receptor-null mice. Neurosci Res. 2015 Jan;90 90-94. doi:10.1016/j.neures.2014.05.003.
- Kanno K, Kokubo H, Takahashi A, Koide T, Ishiura S: Enhanced prepulse inhibition and low sensitivity to a dopamine agonist in HESR1 knockout mice. J Neurosci Res. 2014 Mar;92(3) 287-297. doi:10.1002/jnr.23291.
- Kanno K, Ishiura S: The androgen receptor facilitates inhibition of human dopamine transporter (DAT1) reporter gene expression by HESR1 and HESR2 via the variable number of tandem repeats. Neurosci Lett. 2012 Sep;525(1) 54-59. doi:10.1016/j.neulet.2012.07.021.
- Kanno K, Ishiura S: Differential effects of the HESR/HEY transcription factor family on dopamine transporter reporter gene expression via variable number of tandem repeats. J Neurosci Res. 2011 Apr;89(4) 562-575. doi:10.1002/jnr.22593.
- Kanno K, Shima S, Ishida Y, Yamanouchi K: Ipsilateral and contralateral serotonergic projections from dorsal and median raphe nuclei to the forebrain in rats: immunofluorescence quantitative analysis. Neurosci Res. 2008 Jun;61(2) 207-218. doi:10.1016/j.neures.2008.03.001.
Close
Mechanisms of social discrimination and understanding brain systems for individuality from the view of social behaviors in mice

-
Project leader
Graduate School of Agriculture and Life Sciences, The University of Tokyo
Professor Satoshi Kida
Further information
Message from Project leader
Social discrimination is an essential and basic component of social behaviors. However, mechanisms controlling social discrimination still remain unclear. In this study, we will try to identify neural circuits controlling social discrimination and understand mechanisms of social discrimination at the cellular and molecular levels in mice. Furthermore, we will try to understand brain systems to generate individuality from the view of social behaviors.
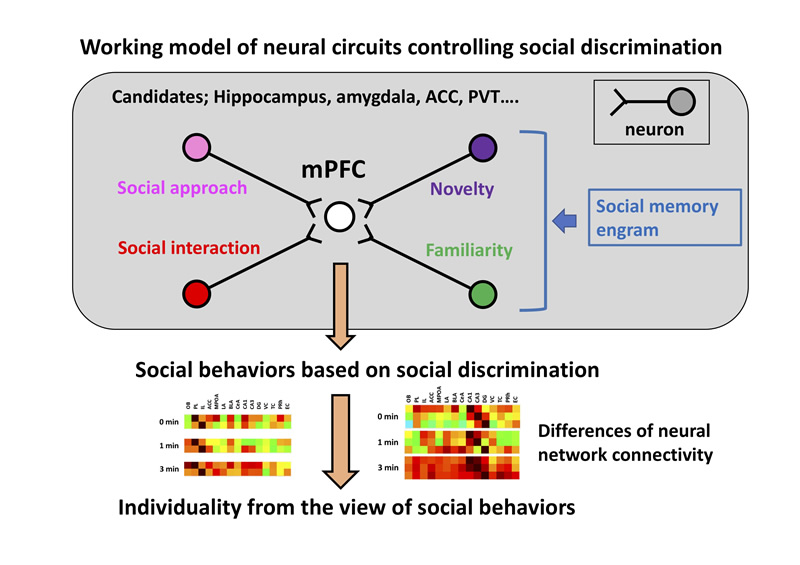
-
Project leader
ProfessorSatoshi KidaGraduate School of Agriculture and Life Sciences, The University of Tokyo
Research Area:
NeuroscienceE-mail:
akida*g.ecc.u-tokyo.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Kida, S. Reconsolidation/Destabilization, Extinction and Forgetting of Fear Memory as Therapeutic Targets for PTSD. Psychopharmacology, 236:49-57.2019. DOI: 10.1007/s00213-018-5086-2
- Serita, T., Miyahara, M., Tanimizu, T., Takahashi, S., Oishi, S., Nagayoshi, T., Tsuji, R., Inoue, H., Uehara, M., Kida, S. Dietary magnesium deficiency impairs hippocampus-dependent memories without changes in the spine density and morphology of hippocampal neurons in mice. Brain. Res. Bull. 2019 149-157.2019. doi: 10.1016/j.brainresbull.2018.11.019.
- Tanimizu, T., Kenney., J.W., Okano, E., K. Kadoma., K, Frankland., P.W., Kida, S. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J. Neurosci., 37, 4103-4116, 2017
- Ishikawa, R., Fukushima, H., Frankland, P.W., Kida, S. Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval eLife, 5: e17464, 2016. doi: 10.7554/eLife.17464.
- Fukushima, H., Zhang, Y., Archbol, G., Ishikawa, R., Nader, K. Kida, S. Enhancement of fear memory by retrieval through reconsolidation. eLife, 3, e02736, 2014
- Suzuki, A., Fukushima, H., Mukawa, T., Toyoda, H., Wu, L-J., Zhao, M-G., Hui Xu, H., Shang, Y., Endoh, K., Iwamoto, Mamiya, N., Okano, E., Hasegawa, H., Mercaldo, V., Yue Zhang, Y., Maeda, R., Ohta, M., Josselyn, S.A., Zhuo, M., & Kida, S. Up-regulation of CREB-mediated transcription enhances both short- and long-term memory. J. Neurosci. 31, 8786-8802 (2011)
- Mamiya, N., Fukushima, H., Suzuki, A., Matsuyama, Z., Homma, S., Frankland, P.W. & Kida, S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J. Neurosci. 29, 402-13 (2009)
- Fukushima, H., Maeda, R., Suzuki, R., Suzuki, A., Nomoto, M., Toyoda, H., Wu, L.-J., Xu, H., Zhao, M.-G., Ueda, K., Kitamoto, K., Mamiya, N., Yoshida, T., Homma, S., Masushige, S., Zhuo, M. & Kida, S. Up-regulation of CaMKIV improves memory formation and rescues memory loss with aging. J. Neurosci. 28, 9910-19 (2008).
- Suzuki, A., Josselyn S., Frankland P., Masushige, S., Silva, A.J. & Kida, S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci., 24, 4787-4795 (2004)
- Kida, S., Josselyn, S., Peña de Ortiz, S., Kogan, J., Masushige, S. & Silva, A.J. CREB required for the stability of new and reactivated fear memory. Nature Neurosci., 5, 348-355 (2002)
Close
Behavior analysis of offspring produced from spermatogonial stem cell transplantation

-
Project leader
Department of Molecular Genetics, Graduate School of Medicine, Kyoto University
Professor Takashi Shinohara
Further information
Message from Project leader
Spermatogonial stem cells are the only stem cells in the gremline that have self-renewal potential. We have succeeded in genetically modifying the male germline using cultured SSCs, designated as gremline stem (GS) cells.This technology based on GS cells and spermatogonial transplantation provides an alternative approach in genetic manipulation of many animal species, in which conventional ES cell-based approach is not applicable (Nat Med 19: 958, 2013; Neuron 86: 617, 2015; A 19: 1124, 2016).
On the other hand, the impact of manipulating SSCs on the health of offspring has not been analyzed to date. Recent studies suggest several lines of evidence regarding the epigenetic inheritance. Given such new studies, it is possible that offspring derived from transplantation of genetically manipulated GS cells may not be normal, which may become an important impediment for future application of this technology to other animal species.
In this project, we will perform behavioral analysis of offspring derived from cultured SSCs and assess the impact of GS cell culture on the next generation.
-
Project leader
ProfessorTakashi ShinoharaDepartment of Molecular Genetics, Graduate School of Medicine, Kyoto University
Research Area:
Reproductive biologyE-mail:
tshinoha*virus.kyoto-u.ac.jp
(Please convert "*" into "@".)URL:
http://www2.mfour.med.kyoto-u.ac.jp/~molgen/index_e.html
Selected Publication
- Watanabe S, Kanatsu-Shinohara M, Ogonuki N, Matoba S, Ogura A, Shinohara T: In vivo genetic manipulation of spermatogonial stem cells and their microenvironment by adeno-associated virus. Stem Cell Reports, 10, 1551-1564, 2018
- Shinohara T., Kazuki K, Ogonuki N, Morimoto H, Matoba S, Hiramatsu K, Honma K, Suzuki T, Hara T, Ogura A, Oshimura M, Kanatsu-Shinohara M, Kazuki Y:Transfer of a mouse artificial chromosome into spermatogonial stem cells generates transchromosomic mice. Stem Cell Reports, 9, 1180-1191, 2017
- Tanaka T, Kanatsu-Shinohara M, Lei Z, Rao CV, Shinohara T: The luteinizing hormone-testosterone pathway regulates mouse spermatogonial stem cell self-renewal by suppressing Wnt5a expression in Sertoli cells. Stem Cell Reports, 7, 279-291,2016
- Kanatsu-Shinohara M, Tanaka T, Ogonuki N, Ogura A, Morimoto H, Cheng PF, Eisenman RN, Trumpp A, Shinohara T: Myc/Mycn-mediated glycolysiss enhances mouse spermatogonial stem cell self-renewal. Genes Dev, 30, 2637-2648, 2016
- Kanatsu-Shinohara M, Naoki H, and Shinohara T: Nonrandom germline transmission of mouse spermatogonial stem cells. Dev Cell, 38, 248-261,2016.
- Takashima S, Kanatsu-Shinohara M, Tanaka T, Morimoto H, Inoue K, Ogonuki N, Jijiwa M, Takahashi M, Ogura A, Shinohara T: Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Reports, 4, 489-502, 2015
- Ishii K, Ishiai M, Morimoto H, Kanatsu-Shinohara M, Niwa O, Takata M, Shinohara T: Trp53-Trp53inp1-Tnfrsf10b pathway regulates the radiation response of mouse spermatogonial stem cells. Stem Cell Reports, 3, 676-689, 2014
- Kanatsu-Shinohara M, Onoyama I, Nakayama KI, Shinohara T: Skp1-Cullin-F-box (SCF)-type ubiquitin ligase FBXW7 negatively regulates spermatogonial stem cell self-renewal. Proc Natl Acad Sci USA, 111, 8826-8831, 2014
- Takashima S, Hirose M, Ogonuki N, Ebisuya M, Inoue K, Kanatsu-Shinohara M, Tanaka T, Nishida E, Ogura A, Shinohara T: Regulation of pluripotency in male germline stem cells by Dmrt1.Genes Dev, 27, 1949-1958, 2013
- Morimoto H, Iwata K, Ogonuki N, Inoue K, Ogura A, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T: ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell, 12, 774-786, 2013
Close
Physiological function of NPBWR1 in individual difference of emotional response.

-
Project leader
Faculty of Medicine, University of Tsukuba
Assistant Professor Shingo Soya
Further information
Message from Project leader
Social contact to the conspecifics is essential for building personal relationships, and it elicits stress responses such as increased heart rate and expression of positive and negative emotion. To understand the diversity in interpersonal distance, it is important to focus on the individual difference in emotional responses. NPBWR1, a receptor binds with neuropeptide B/W has reported to be involved in fear, autonomic response and social behavior. Investigating the physiological role of NPBWR1 and neural circuit it governs could reveal the individual difference in emotional response which affects interpersonal distance to regulate social contact.
-
Project leader
Assistant ProfessorShingo SoyaFaculty of Medicine, University of Tsukuba
Research Area:
Behavioral physiology, Molecular neuroscienceE-mail:
soya.shingo.gp*u.tsukuba.ac.jp
(Please convert "*" into "@".)URL:
https://wpi-iiis.tsukuba.ac.jp/research/member/detail/takeshisakurai/
Selected Publication
- Shota Kodani*, Shingo Soya*, Takeshi Sakurai: Optogenetic manipulation of neural circuits during monitoring sleep/wakefulness states in mice. JOVE, in press. *Equal contribution.
- Shingo Soya and Takeshi Sakurai: Orexin as a modulator of fear-related behavior; Hypothalamic control of noradrenaline circuit. Brain Research, 2018
- Yibing Wang, Liqin Cao, Chia-Ying Lee, Tomohiko Matsuo, Kejia Wu, Greg Asher, Lijun Tang, Tsuyoshi Saitoh, Jamie Russell, Daniela Klewe-Nebenius, Li Wang, Shingo Soya, Emi Hasegawa, Yoan Chérasse, Jiamin Zhou, Yuwenbin Li, Tao Wang, Xiaowei Zhan, Chika Miyoshi, Yoko Irukayama, Jie Cao, Julian P Meeks, Laurent Gautron, Zhiqiang Wang, Katsuyasu Sakurai, Hiromasa Funato, Takeshi Sakurai, Masashi Yanagisawa, Hiroshi Nagase, Reiko Kobayakawa, Ko Kobayakawa, Bruce Beutler, Qinghua Liu: Large-scale forward genetics screening identifies Trpa1 as a chemosensor for predator odor-evoked innate fear behaviors. Nature Communications, 9, 2018
- Shingo Soya, Tohru T Takahashi, Thomas Mchugh, Takashi Maejima, Abe manabu, Shinji Sakimura, Takeshi Sakurai: Orexin modulates behavioral fear expression through locus coeruleus. Nature Communications, 8, 1-14, 2017
- Shota Kodani, Shingo Soya, Takeshi Sakurai: Excitation of GABAergic neurons in the bed nucleus of the stria terminalis triggers immediate transition from non-rapid eye movement sleep to wakefulness in mice. Journal of Neuroscience, 37, 7174-7176, 2017
- MD Golam Abbas, Hirotaka Shoji, Shingo Soya, Mari Hondo, Tsuyoshi Miyakawa, Takeshi Sakurai: Comprehensive behavioral analysis of Ox1r-/- mice showed implication of orexin receptor-1 in mood, anxiety and social behavior. Frontiers in behavioral neuroscience, 9, 324, 2015
- Takashi Matsui, Shingo Soya, Kentaro Kawanaka, Hideaki Soya: Brain glycogen decreases during intense exercise without hypoglycemia: The possible involvement of serotonin. Neurochemical Research, 40 (7), 1333-1340, 2015
- Shingo Soya, Hirotaka Shoji, Emi Hasegawa, Mari Hondo, Tsuyoshi Miyakawa, Masashi Yanagisawa, Michihiro Mieda, and Takeshi Sakurai: Orexin Receptor-1 in the Locus Coeruleus Plays an Important Role in Cue-Dependent Fear Memory Consolidation. The Journal of Neuroscience, 33 (36): 14549-14557・14549, September4, 2013
Close
The emergence of social individuality in group-living mice

-
Project leader
Department of Physiology, Dokkyo Medical University
Lecturer Kensaku Nomoto
Further information
Message from Project leader
Many animals, including man, live in groups. They socially interact with each other, establish long-term relationships between individuals, and form a complicated social network. Here we define “social individuality” as social behavioral traits, including the position in the social hierarchy and the social role, that are acquired through social interactions. In this project, using a new tracking system that allows us to monitor mouse social behavior over several days and neuroscientific techniques such as optogenetic and chemogenetic tools that allow us to manipulate neuronal activities of a specific cell type in a reversible manner, we will test the hypothesis that a history of social interactions shapes “social individuality”. Specifically, we will examine the role of oxytocin on the emergence of “social individuality”.
-
Project leader
LecturerKensaku NomotoDepartment of Physiology, Dokkyo Medical University
Research Area:
Behavioral NeuroscienceE-mail:
nomoto*dokkyomed.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Nomoto K, Ikumi M, Otsuka M, Asaba A, Kato M, Koshida N, Mogi K, Kikusui T: Female mice exhibit both sexual and social partner preferences for vocalizing males. Integrative zoology, 13, 735-744, 2018.
- Lak A, Nomoto K, Keramati M, Sakagami M, Kepecs A: Midbrain Dopamine Neurons Signal Belief in Choice Accuracy during a Perceptual Decision. Current Biology, 27, 821-832, 2017.
- Nomoto K, Lima SQ: Enhanced male-evoked responses in the ventromedial hypothalamus of sexually receptive female mice. Current Biology, 25, 589-594, 2015.
- Nomoto K, Schultz W, Watanabe T, Sakagami M: Temporally extended dopamine responses to perceptually demanding reward-predictive stimuli. Journal of Neuroscience, 30, 10692-10702, 2010.
- Kobayashi S, Nomoto K, Watanabe M, Hikosaka O, Schultz W, Sakagami M: Influences of Rewarding and Aversive Outcomes on Activity in Macaque Lateral Prefrontal Cortex. Neuron, 51, 861-870, 2006.
Close
Neural networks underlying individual differences in sensitivity to others’ rewards of the macaque

-
Project leader
Division of Behavioral Development, Department of System Neuroscience, National Institute for Physiological Sciences
Assistant Professor Atsushi Noritake
Further information
Message from Project leader
We humans are inherently social and therefore our behaviors are modulated by rewards not only to oneself (self-rewards) but also to others (other-rewards). The degree of behavioral modulation varies across individuals, which could be attributed to variable degree of sensitivity in response to other-rewards. Our previous study using monkeys unraveled the neural representation and computation of self- and other-rewards in the brain (Noritake et al., 2018); (1) the self-reward information and other-reward information were monitored in different subpopulations in the medial prefrontal cortex (MPFC), a core node in the processing of social information; (2) a subjective reward (anintegration of self- and other-rewards) was encoded in the dopaminergic (DA) midbrain nuclei, a crucial node in the processing of reward information; and (3) a causal analysis of local field potentials revealed that the neuronal information flow was predominantly from the MPFC to the DA midbrain nuclei, suggesting the computation of subjective rewards as an integration of self- and other-rewards. However, it remains to be answered what neural substrates cause such individual variability in sensitivity to other-rewards. The proposed study extends our previous findings by investigating how the neuronal modulations in the MPFC and the DA midbrain nuclei reflect individual differences in sensitivity to other-rewards. Further, we hypothesize that the processing of other-rewards is impaired in autistic individuals, and hence focus on neural differences between monkeys with normal development and monkeys with an autistic phenotype. Our study has potential to elucidate the neural mechanisms underlying individual differences in sensitivity to other-rewards and to bridge a gap between the microscopic mechanism (molecular and genetic studies using rodents) and the macroscopic understanding (behavioral, cognitive, and brain functional imaging studies in humans).
-
Project leader
Assistant ProfessorAtsushi NoritakeDivision of Behavioral Development, Department of System Neuroscience, National Institute for Physiological Sciences
Research Area:
system neuroscience, experimental psychologyE-mail:
noritake*nips.ac.jp
(Please convert "*" into "@".)URL:
https://www.nips.ac.jp/eng/research/group/post-48/
https://www.nips.ac.jp/dbd/dbd_eng/index.html
Selected Publication
- Noritake A, Nakamura K., Encoding prediction signals during appetitive and aversive Pavlovian conditioning in the primate lateral hypothalamus. J Neurophysiol. 121(2):396-417, 2019.
- Noritake A, Ninomiya T, Isoda M., Social reward monitoring and valuation in the macaque brain. Nat Neurosci. 21(10):1452-1462, 2018.
- Isoda M, Noritake A, Ninomiya T., Development of social systems neuroscience using macaques. Proc Jpn Acad Ser B Phys Biol Sci. 94(7):305-323, 2018.
- Ninomiya T, Noritake A, Ullsperger M, Isoda M. Performance monitoring in the medial frontal cortex and related neural networks: From monitoring self actions to understanding others' actions. Neurosci Res. pii: S0168-0102(18)30080-4, 2018.
- Ueda Y, Yamanaka K, Noritake A, Enomoto K, Matsumoto N, Yamada H, Samejima K, Inokawa H, Hori Y, Nakamura K, Kimura M., Distinct Functions of the Primate Putamen Direct and Indirect Pathways in Adaptive Outcome-Based Action Selection. Front neuroanat 11:66, 2017.
- Higuchi T, Ishizaki Y, Noritake A, Yanagimoto Y, Kobayashi H, Nakamura K, Kaneko K. Spatiotemporal characteristics of gaze of children with autism spectrum disorders while looking at classroom scenes. PLoS One.12(5):e0175912, 2017.
- Isoda M, Noritake A. What makes the dorsomedial frontal cortex active during reading the mental states of others? Front Neurosci.7:232, 2013.
Close
Elucidating the role of brain- and mammal-specific genes in the formation of individual differences

-
Collaborator
Molecular and Cellular Biology, Medical Institute of Bioregulation, Kyushu University
Associate Professor Akinobu Matsumoto
Further information
Message from Collaborator
Although one of the criteria for classification of long non-coding RNA is that no ORF should encode more than 100 amino acids, short predicted ORFs (less than 100 amino acids) can be found in many lncRNA molecules. Recently, we have identified a set of novel polypeptides that are expressed from short predicted ORFs within putative lncRNAs by mass-spectrometry, and found the biological functions for one of the polypeptides [Matsumoto et al., Nature 541: 228-232 (2017)].
We have identified more novel polypeptides by applying in silico approach. Interestingly, many of the polypeptides specifically expressed in the brain, and exist only in mammals. We thus hypothesized that these brain- and mammal-specific polypeptides are required for higher brain functions and the formation of individual differences.
-
Collaborator
Associate ProfessorAkinobu MatsumotoMolecular and Cellular Biology, Medical Institute of Bioregulation, Kyushu University
Research Area:
Molecular biologyE-mail:
akinobu*bioreg.kyushu-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Matsumoto A, Nakayama KI: Hidden Peptides Encoded by Putative Noncoding RNAs. Cell Struct. Funct., 18;43(1):75-83 (2018).
- Matsumoto A, Clohessy JG, Pandolfi PP: SPAR, a lncRNA encoded mTORC1 inhibitor. Cell Cycle, 541(7636):1-2 (2017).
- Matsumoto A, Pasut A, Matsumoto M, Yamashita R, Fung J, Monteleone E, Saghatelian A, Nakayama KI, Clohessy JG, Pandolfi PP: mTORC1 and muscle regeneration are regulated by the LINC00961 encoded SPAR polypeptide. Nature, 12;541(7636):228-232 (2017).
- Matsumoto A, Takeishi S, Nakayama KI: p57 regulates T cell development and prevents lymphomagenesis by balancing p53 activity and pre-TCR signaling. Blood, 29;123(22):3429-3439 (2014).
- Matsumoto A, Nakayama KI: Role of key regulators of the cell cycle in maintenance of hematopoietic stem cells. Biochem. Biophys. Acta, 1830: 2335-2344 (2013).
- Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y, Nakayama K, Nakayama KI: p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell, 9: 262-271 (2011).
Close
The neural mechanism whereby experience shapes individual differences in psychobehavioral traits

-
Project leader
National Institute of Information and Communications Technology, Advanced ICT Research Institute
Executive Researcher Daisuke Yamamoto
Further information
Message from Project leader
We aim at elucidating how genetic and environmental factors shape male sexual orientation in the fruit fly Drosophila, with special reference to the social-experience-dependent plasticity in neurons.
-
Project leader
Executive ResearcherDaisuke YamamotoNational Institute of Information and Communications Technology, Advanced ICT Research Institute
Research Area:
behavioral genetics, neurogeneticsE-mail:
daichan*nict.go.jp
(Please convert "*" into "@".)
Selected Publication
- Sato, K., Ito, H., Yokoyama, A., Toba, G. and Yamamoto, D.: Partial proteasomal degradation of Lola triggers the male-to-female switch of a dimorphic courtship circuit. Nature Commun. 10, 166.(2019)
- Chowdhury, Z. S., Sato, K. and Yamamoto, D.: The core-promoter factor Trf2 mediates a Fruitless action to masculinize neurobehavioral traits in Drosophila. Nature Commun. 8, 1480.(2017)
- Kohatsu, S. and Yamamoto, D.: Visually induced initiation of Drosophila innnate courtship-like following pursuit is mediated by central excitatory state. Nature Commun. 6, 6457. (2015)
- Hamada-Kawaguchi, N., Nore, B. F., Kuwada, Y., Smith C. I. E., and Yamamoto, D. : Btk29A promotes Wnt4 signaling in the niche to terminate germ cell proliferation in Drosophila. Science 343, 294-297.(2014)
- Yamamoto, D. and Koganezawa, M.: Genes and circuits of courtship behaviour in Drosophila males. Nature Rev. Neurosci. 14, 681-692. (2013)
- Ito, H., Sato, K., Koganezawa, M., Ote, M., Matsumoto, K., Hama, C. and Yamamoto, D.: Fruitless cooperates with two antagonistic chromatin factors to establish single-neuron sexual dimorphism. Cell 149, 1327-1338.(2012)
- Kohatsu, S., Koganezawa, M. and Yamamoto, D.: Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in a Drosophila male. Neuron 69, 498-508.(2011)
- Kimura, K.-I., Hachiya, T., Koganezawa, M., Tazawa, T. and Yamamoto, D.: Fruitless and Doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759-769.(2008)
- Kimura, K.-I., Ote, M., Tazawa, T. and Yamamoto, D.: fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438, 229-233.(2005)
- Usui-Aoki, K., Ito, H., Ui-Tei, K., Takahashi, K. Lukacsovich, T., Awano, W., Nakata, H., Piao, Z. F., Nilsson, E., Tomida, J. and Yamamoto, D.: Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nature Cell Biol. 2, 500-506.(2000)
Close
Molecular/neural basis underlying individual differences in the boldness using new closed colony.

-
Project leader
Faculty of Pharmaceutical Sciences, Hokkaido University
Assistant Professor Saori Yokoi
Further information
Message from Project leader
The boldness and the shyness were studied as personality traits in many animals from insects to mammalians. However, the gene regulating this personality have not been identified in any species. To address this issue, we did the open-field test and measured boldness among several novel closed-colony medaka established from wild population in western Setouchi area. The greatest difference in the boldness was detected between two population, #1 and #2. Therefore, using these medaka, we aim to find novel gene required for emerging individuality of the boldness. As the genome size of medaka is only a quarter of those of mice and humans, we expect that it is easier to identify such genes in medaka than in mammalians. Our study may shed light on the evolutional origin of molecular/neural basis underlying individual differences in the boldness.
-
Project leader
Assistant ProfessorSaori YokoiFaculty of Pharmaceutical Sciences, Hokkaido University
Research Area:
Behavioral genetics, Molecular biologyE-mail:
yokois*pharm.hokudai.ac.jp
(Please convert "*" into "@".)URL:
https://sites.google.com/rnabiol.com/home/home?authuser=0
Selected Publication
- Taiwa K, Yokoi S, Mito M, Fujii K, Kimura Y, Iwasaki S, Nakagawa S: UPA-Seq: Prediction of Functional LncRNAs Using Differential Sensitivity to UV Crosslinking. RNA (New York, N.Y.), 24(12), 1785-1802, 2018.
- Watakabe I, Hashimoto H, Kimura Y, Yokoi S, Naruse K, Higashijima SI: Highly efficient generation of knock-in transgenic medaka by CRISPR/Cas9-mediated genome engineering. Zoological letters, 4, 2018.
- *Okuyama T, *Yokoi S, Takeuchi H: Molecular basis of social competence in medaka fish. Development, growth & differentiation, 59(4), 211-218, 2017.
- Yokoi S, Ansai S, Kinoshita M, Naruse K, Kamei Y, Young LJ, Okuyama T, Takeuchi H: Mate-guarding behavior enhances male reproductive success via familiarization with mating partners in medaka fish, Frontiers in Zoology, 13, 21, 2016.
- Isoe Y, Konagaya Y, Yokoi S, Kubo T, Takeuchi H: Ontogeny and Sexual Differences in Swimming Proximity to Conspecifics in Response to Visual Cues in Medaka Fish
Zoological Science, 33(3), 246-254, 2016. - Yokoi S, Okuyama T, Kamei Y, Naruse K, Taniguchi Y, Ansai S, Kinoshita M, Young LJ, Takemori N, Kubo T, Takeuchi H: An Essential Role of the Arginine Vasotocin System in Mate-Guarding Behaviors in Triadic Relationships of Medaka Fish (Oryzias latipes). PLoS genetics, 11, e1005009, 2015.
- Okuyama T, Yokoi S, Abe H, Isoe Y, Suehiro Y, Imada H, Tanaka M, Kawasaki T, Yuba S, Taniguchi Y, Kamei Y, Okubo K, Shimada A, Naruse K, Takeda H, Oka Y, Kubo T, Takeuchi H: A neural mechanism underlying mating preferences for familiar individuals in medaka fish. Science (New York, N.Y.), 343, 91-94, 2014.
Close
The transcriptional regulation for regulating individual difference in the vocal learning bias.

-
Project leader
Department of Biology, Faculty of Science, Hokkaido University
Associate Professor Kazuhiro Wada
Further information
Message from Project leader
Individual differences in vocal learning ability are widely acknowledged, yet little is known about the factors that underlie them. Learning is influenced by not only the environment but also the genetical feature in individuals. Although offspring carry two chromosomal sets from two-parents, which are not sequentially identical, recently, increasing evidences point the existence of uneven parental genomic contribution to the offspring’s phenotypic development. However, it remains unknown how two-parental genetic features interact and further affect offspring’s learning. Here we investigate the parental genetic contribution to offspring’s vocal learning using hybrid songbirds, of which two-parental species produce distinctive species-specific songs.
-
Project leader
Associate ProfessorKazuhiro WadaDepartment of Biology, Faculty of Science, Hokkaido University
Research Area:
Behavioral neurobiology, Molecular neuroscienceE-mail:
wada*sci.hokudai.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Hayase S, Wang H, Ohgushi E, Kobayashi M, Mori C, Horita H, Mineta K, Liu WC, Wada K.
Vocal practice regulates singing activity-dependent genes underlying age-independent vocal learning in songbirds.
PLoS Biol. 16(9): e2006537. 2018 - Hayase S, Wada K.
Singing activity-driven Arc expression associated with vocal acoustic plasticity in juvenile songbird.
Eur J Neurosci. 48(2):1728-1742. 2018 - Mori C, Liu WC, Wada K.
Recurrent development of song idiosyncrasy without auditory inputs in the canary, an open-ended vocal learner.
Scientific Reports 8:8732. 2018 - Sato D, Mori C, Sawai A, Wada K.
Familial bias and auditory feedback regulation of vocal babbling patterns during early song development
Scientific Reports 6:30323. 2016 - Imai R, Sawai A, Hayase S, Furukawa H, Asogwa CN, Sanchez M, Wang H, Mori C, Wada K.
A quantitative method for analyzing species-specific vocal sequence pattern and its developmental dynamics.
Journal of Neuroscience Methods 271:25-33. 2016 - Ohgushi E, Mori C, Wada K.
Diurnal oscillation of vocal development associated with clustered singing by juvenile songbirds.
Journal of Experimental Biology 218: 2260-2268. 2015 - Mori C, Wada K.
Audition-independent vocal crystallization associated with intrinsic developmental gene expression dynamics.
Journal of Neuroscience 35:878-89. 2015. - Jarvis ED, Yu J, Rivas MV, Horita H, Feenders G, Whitney O, Jarvis S, Jarvis ER, Kubikova L, Puck AE, Siang-Bakshi C, Martin S, McElroy M, Hara E, Howard J, Mouritsen H, Chen CC*, Wada K.
A global view of the functional molecular organization of the avian cerebrum: Mirror images and functional columns.
Journal of Comparative Neurology 521:3614-3665. 2013 - Liu WC, Wada K, Jarvis ED, Nottebohm F
Rudimentary substrates for vocal learning in a suboscine.
Nature Communication 4:2082. 2013 - Wada K, Hayase S, Imai R, Mori C, Kobayashi M, Liu WC, Takahasi M, Okanoya K.
Differential androgen receptor expression and DNA methylation state in striatum song nucleus Area X between wild and domesticated songbird strains.
European Journal of Neuroscience 38:2600-2610. 2013 - Wada K, Howard JT, McConnell P, Whitney O, Lints T, Rivas MV, , Horita H, Patterson MA, White SA, Scharff C, Haesler S, Zhao S, Sakaguchi H, Hagiwara M, Shiraki T, Hirozane-Kishikawa T, Skene P, Hayashizaki Y, Carninci P, Jarvis ED*
A molecular neuroethological approach for identifying and characterizing a cascade of behaviorally regulated genes.
PNAS 103:15212-15217. 2006
Close
RNA epigenetics as a new interface between genome and environment for the emergence of individuality

-
Project leader
Institute for integrated cell-material sciences (iCeMS), Kyoto University
Associate Professor Dan Ohtan Wang
Further information
Message from Project leader
Recently, epitranscriptomics, a term representing >150 natural RNA modifications in all life domains and their regulation have come into intense research focus as a largely unexplored post-transcriptional regulatory mechanism. In this project, we will utilize a rich toolbox including the most advanced RNA modification detection methods with next-generation sequencing techniques, bioinformatics, biochemical analysis, cell biology, transgenic techniques, and behavior analysis, to identify a new mechanistic interface between genes and experience, to understand the emergence of cognitive individual differences.
-
Project leader
Associate ProfessorDan Ohtan WangInstitute for integrated cell-material sciences (iCeMS), Kyoto University
Research Area:
Molecular neuroscience, RNA biologyE-mail:
dwang*icems.kyoto-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Merkurjev D, Hong WT, Iida K, Goldie BJ, Yamaguti H, Oomoto I, Ohara T, Kawaguchi S, Hirano T, Martin KC, Pellegrini M, Wang DO*. Synaptic N6 methyladenosine (m6A) reveals functional partitioning of localized transcripts. Nature Neuroscience, 21, 1004–1014 (2018).
- Wang DO*. Live Imaging of Nuclear RNPs in Mammalian Complex Tissue with ECHO-liveFISH. Methods Mol Biol. (2018); 1649:259-272. doi: 10.1007/978-1-4939-7213-5_17.
- Oomoto I, Hirano-Suzuki A, Umeshima H, Han YW, Yanagisawa H, Carlton P, Harada Y, Kengaku M, Okamoto A, Shimogori T, and Wang DO*. ECHO-liveFISH: in vivo RNA Labeling Reveals Dynamic Regulation of Nuclear RNA Foci in Living Tissues. Nucl Acids. Res doi:10.1093/narlgkv614 (2015) * This work has been featured in “Nikkei Sangyo Shimbun” and “Weekly Economist”.
- Wang DO and Akimitsu Okamoto*. ECHO probes: fluorescence emission control for nucleic acid imaging. Journal of Photochemistry and Photobiology, C: Photochemistry Reviews 13(2): 112-123 (2012)
- Meer E#, Wang DO#, Kim SM, Barr I, Guo F, and Martin KC. Identification of a cis-element that localizes mRNA to synapses. PNAS 109 (12): 4639-44 (2012) (#Equal contribution)
- Wang DO*, Hitomi Matsuno, Shuji Ikeda, Hiroyuki Yanagisawa, Yasunori Hayashi, and Akimitsu Okamoto*. A quick and simple FISH protocol with hybridization-sensitive fluorescent linear oligodeoxynucleotide probes. RNA (2012) Jan;18(1):166-75
- Wang DO, Martin KC*, and Zukin RS*. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. (2010) 33(4):173-82
- Wang DO, Kim SM, Zhao Y, Hwang HG, Miura SK, Sossin WS, and Martin KC*. Synapse- and stimulus-specific local translation during long-term neuronal plasticity. Science. (2009) 324(5934): 1536-40.
- Wang D and Quick MW*. Trafficking of the plasma membrane GABA transporter GAT1: Size and rates of an acutely recycling pool. J Biol Chem. (2005) 280:18703-18709.
Close
A03: Tools and theories for generative brain systems for individuality
Quantitative analysis of the activities of the transcription factors that generates animal’s personality

-
Project leader
Graduate School of Life Sciences, Tohoku University
Professor Kentaro Abe
Further information
Message from Project leader
The development of the personality of an animal is influenced both by intrinsic and extrinsic factors. Transcription factors regulate the expression of the genetic information according to postnatal experience, thus, may function as a molecular hub to integrate the influence of intrinsic and extrinsic factors. Through using the original method that allows quantitative analysis of the activity of the transcription factors in the brain, this research aims to reveal the mechanisms that affect the development of personalities in animals.
-
Project leader
ProfessorKentaro AbeGraduate School of Life Sciences, Tohoku University
Research Area:
Developmental Neuroscience, Molecular NeuroscienceE-mail:
k.abe*tohoku.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Abe K, Matsui S. and Watanabe D. Transgenic songbirds with suppressed or enhanced activity of CREB
transcription factor. Proc Natl Acad Sci U.S.A. 112(24):7599-604, 2015. - Abe K and Watanabe D. Songbirds possess the spontaneous ability to discriminate syntactic rules. Nature Neuroscience, 14(8):1067-73, 2011.
- Abe K and Takeichi M. EPLIN mediates linkage of the cadherin-catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U.S.A. 105(1):13-9, 2008.
- Abe K and Takeichi M. NMDA receptor activation induces calpain-mediated β-catenin cleavages for triggering gene expression. Neuron, 53(3):387-97, 2007.
- Abe K, Chisaka O, van Roy F. and Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by αN-catenin. Nature Neuroscience, 7(4):357-63, 2004.
- Togashi H., Abe K, Mizoguchi A., Takaoka K., Chisaka O. and Takeichi M. Cadherin Regulates Dendritic Spine Morphogenesis. Neuron, 35, 77-89, 2002.
Close
Generation of humanized mouse models via genome editing to elucidate molecular basis for neurodevelopmental diversities

-
Project leader
Department of Biochemistry and Cellular Biology, National Institute of Neuroscience, National Center of Neurology and Psychiatry
Research Fellow Yukiko U. Inoue
Further information
Message from Project leader
For our integrative research project toward understanding individuality, model animals play an essential role to examine the effects of changes in genetic/environmental factors on brain activity and behavior. We focus on a peptide hormone, oxytocin, and manipulate its receptor gene in mouse brains via CRISPR/Cas9 genome editing technology to elucidate the relationship between individual genetic variations and social behavior. We also share our genome editing techniques with the group members by rapidly generating genome-edited mouse models.
-
Project leader
Research FellowYukiko U. InoueDepartment of Biochemistry and Cellular Biology, National Institute of Neuroscience, National Center of Neurology and Psychiatry
Research Area:
Genome editing, Neurodevelopmental disorderE-mail:
yinn3*ncnp.go.jp
(Please convert "*" into "@".)URL:
https://www.ncnp.go.jp/nin/guide/r6/index-lab2/Eng/pg235.html
Selected Publication
- Inoue YU, Morimoto Y, Hoshino M, Inoue T: Generation of Pax6-IRES-EGFP
knock-in mouse via the cloning-free CRISPR/Cas9 system to reliably visualize neurodevelopmental dynamics. Neuroscience Research, 132, 1-7, 2018 (chosen as the cover article) - Inoue YU, Inoue T: Brain enhancer activities at the gene-poor 5p14.1 autism-associated locus. Scientific Reports, 6, 31227, 2016
- Ito Y, Inoue N, Inoue YU (equally contributed first author), Nakamura S, Matsuda Y, Inagaki M, Ohkubo T, Asami J, Terakawa YW, Kohsaka S, Goto Y, Akazawa C, Inoue T, Inoue K: Additive dominant effect of a SOX10 mutation underlies a complex phenotype of PCWH. Neurobiology of Disease, 80, 1-14, 2015 (chosen as the cover article)
- Owa T, Taya S, Miyashita S, Yamashita M, Adachi T, Yamada K, Yokoyama M, Aida S, Nishioka T, Inoue YU, Goitsuka R, Nakamura T, Inoue T, Kaibuchi K, Hoshino M: Meis1 coordinates cerebellar granule cell development by regulating Pax6 transcription, BMP signaling and Atoh1 degradation. Journal of Neuroscience, 38, 1277-1294, 2018
- Hass MR, Liow HH, Chen X, Sharma A, Inoue YU, Inoue T, Reeb A, Martens A, Fulbright M, Raju S, Stevens M, Boyle S, Park JS, Weirauch MT, Brent MR, Kopan R: SpDamID: Marking DNA bound by protein complexes identifies Notch-dimer responsive enhancers. Molecular Cell, 59, 685-697, 2015
Close
Investigating the adaptive functions of individuality by using mathematical models of evolution and behaviors.

-
Project leader
Department of Psychology, Graduate School of Informatics, Nagoya University, Nagoya, Aichi, 464-8601, Japan.
Associate Professor Kentaro Katahira
Further information
Message from Project leader
Substantial individual differences in behavioral tendency have been observed in wide-range of animal species as well as in humans. Recently, such behavioral individuality is reported to emerge even with identical genetic and environmental conditions. In addition, there are often qualitative, rather than quantitative, individual differences in behavioral tendencies. In this project, we investigate the adaptive function of such behavioral individuality through theoretical consideration based on mathematical modeling of behavior and evolution.
-
Project leader
Associate ProfessorKentaro KatahiraDepartment of Psychology, Graduate School of Informatics, Nagoya University, Nagoya, Aichi, 464-8601, Japan.
Research Area:
Computational behavioral science, Psychology of learningE-mail:
katahira.kentaro*b.mbox.nagoya-u.ac.jp
(Please convert "*" into "@".)URL:
http://profs.provost.nagoya-u.ac.jp/view/html/100007097_en.html
Selected Publication
- Katahira K.: The statistical structures of reinforcement learning with asymmetric value updates. Journal of Mathematical Psychology, 87, 31-45, 2018.
- Katahira K, & Yamashita Y: A theoretical framework for evaluating psychiatric research strategies. Computational Psychiatry, 1, 184-207, 2017.
- Katahira K, Yuki S, Okanoya K: Model-based estimation of subjective values using choice tasks with probabilistic feedback. Journal of Mathematical Psychology, 79, 29–43,2017.
- Toyama A., Katahira K, Ohira H: A simple computational algorithm of model-based choice preference. Cognitive, Affective, & Behavioral Neuroscience, 17, 764–783, 2017
- Katahira K: How hierarchical models improve point estimates of model parameters at the individual level. Journal of Mathematical Psychology, 73, 37-58, 2016.
- Katahira K: The relation between reinforcement learning parameters and the influence of reinforcement history on choice behavior. Journal of Mathematical Psychology, 66, 59–69, 2015.
- Katahira K, Matsuda YT, Fujimura T, Ueno K, Asamizuya T, Suzuki C, Cheng K, Okanoya K, Okada M: Neural basis of decision-making guided by emotional outcomes. Journal of Neurophysiology, 113, 3056-3068. 2015.
Close
Characterizing individuality of human brain by spatio-temporal analysis of non-invasive neuroimaging data

-
Project leader
Advanced Telecommunications Research Institute International
Department Head Motoaki Kawanabe
Further information
Message from Project leader
Recent advances in non-invasive neuroimaging reveal some relationships between ‘individuality' of human brain and behavioral characteristics and psychological traits. However, at present, it is not easy to approach neural bases behind human personality from such indirect measurements of brain activities. In this project, we will further develop machine learning methods to extract latent components of brain activities for two neuroimaging data: electroencephalogram (EEG) and functional magnetic resonance imaging (fMRI). Through the analyses of the latent structures obtained by the developed methods from EEG Microstates and fMRI Common Neural Modes shared among varous brain states, we aim at creating a novel ‘individuality'index based on neuroimaging data.
-
Project leader
Department HeadMotoaki KawanabeAdvanced Telecommunications Research Institute International
Research Area:
Machine Learning, Biomedical EngineeringE-mail:
kawanabe*atr.jp
(Please convert "*" into "@".)
Selected Publication
- Hirayama J, Hyvärinen A, Kawanabe M: SPLICE: Fully tractable hierarchical extension of ICA with pooling. Proc. of the 34th
International Conference on Machine Learning, PMLR 70, 1491-1500, 2017. - Hirayama J, Hyvärinen A, Kiviniemi V, Kawanabe M, Yamashita O: Characterizing variability of modular brain connectivity with constrained principal component analysis. PLoS One, 11, e0168180, 2016.
- Hyvärinen A, Hirayama J, Kiviniemi V, Kawanabe M: Orthogonal connectivity factorization: Interpretable decomposition of variability in correlation matrices.
Neural Computation, 28, 445-484, 2016. - Morioka H, Kanemura A, Hirayama J, Shikauchi M, Ogawa T, Ikeda S, Kawanabe M, Ishii S: Learning a common dictionary for subject-transfer decoding with resting calibration. NeuroImage, 111, 167-178, 2015.
- Morioka H, Kanemura A, Morimoto S, Yoshioka T, Oba S, Kawanabe M, Ishii S: Decoding spatial attention by using cortical currents estimated from electroencephalography with near-infrared spectroscopy prior information. NeuroImage, 90, 128-139, 2014.
- Samek W, Kawanabe M, Müller K-R: Divergence-based framework for common spatial patterns algorithms. IEEE Reviews in Biomedical Engineering, 7, 50-72, 2014.
- Kawanabe M, Samek W, Müller K-R, Vidaurre C: Robust common spatial filters with a maxmin approach. Neural Computation, 26, 349-376, 2014.
- Binder A, Müller K-R, Kawanabe M: On taxonomies for multi-class image categorization.
International Journal of Computer Vision, 99, 281-301, 2012. - Haufe S, Tomioka R, Nolte G, Müller K-R, Kawanabe M: Modeling sparse connectivity between underlying brain sources for EEG/MEG. IEEE Transactions on Biomedical Engineering, 57, 1954-1963, 2010.
- Blankertz B, Tomioka R, Lemm S, Kawanabe M, Müller K-R: Optimizing spatial filters for robust EEG single-trial analysis. IEEE Signal Processing Magazine, 25, 41-56, 2008.
Close
Development and application of synaptic imaging for elucidating mechanism of individuality formation.

-
Project leader
Graduate School of Biostudies, Kyoto University
Project Associate Professor Masayuki Sakamoto
Further information
Message from Project leader
As a new technique to elucidate the mechanism of individuality formation, we will develop an imaging method to observe synaptic activity of neurons during postnatal developmental period.
-
Project leader
Project Associate ProfessorMasayuki SakamotoGraduate School of Biostudies, Kyoto University
Research Area:
Imaging, NeuroscienceE-mail:
sakamoto.masayuki.2e*kyoto-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Bando, Y*#, Sakamoto M*#, Kim S, Ayzenshtat I, Yuste R: Comparative evaluation of genetically encoded voltage indicators. Cell Reports 26, 802-813, 2019.
- Kwon T#, Sakamoto M#, Peterka D.S, Yuste R: Attenuation of synaptic potentials in dendritic spines. Cell Reports 20,1100-1110, 2017.
- Sakamoto M, Ieki N, Miyoshi G, Mochimaru D, Miyachi H, Imura T, Yamaguchi M, Fishell G, Mori K, Kageyama R, Imayoshi I: Continuous postnatal neurogenesis contributes to the formation and maintenance of the functional olfactory bulb neural circuits. Journal of Neuroscience, 34, 5788-5799, 2014.
- Sakamoto M, Kageyama R, Imayoshi I: The functional significance of newly born neurons integrated into olfactory bulb circuits. Frontiers in Neuroscience, 8, 121, 2014.
- Sakamoto M, Imayoshi I, Ohtsuka T, Yamaguchi M, Mori K, Kageyama R: Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proceedings of the National Academy of Sciences, 108: 8479-8484, 2011.
Close
Individual differences in memory and stress-induced physiological responses

-
Project leader
Graduate School of Pharmaceutical Sciences, The University of Tokyo
Associate Professor Takuya Sasaki
Further information
Message from Project leader
Stress-induced responses in animals considerably differ across individuals. We are studying how cortical-wide functional correlations are associated with such individual differences in stress-induced dysfunctions of the peripheral organs. We expect the evidence will advance the understanding of the neurophysiological correlates of mind-body associations in health and disease from the viewpoint of individual differences.
-
Project leader
Associate ProfessorTakuya SasakiGraduate School of Pharmaceutical Sciences, The University of Tokyo
Research Area:
neurophysiology, neuropharmacologyE-mail:
tsasaki*mol.f.u-tokyo.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Aoki Y, Igata H, Ikegaya Y, Sasaki T: Integration of goal-directed signals onto spatial maps of hippocampal place cells. Cell Reports, 2019
- Konno D, Nakayama R, Tsunoda M, Funatsu T, Ikegaya Y, Sasaki T: Collection of biochemical samples with brain-wide electrophysiological recordings from a freely moving rodent. Journal of Pharmacological Sciences, 2019
- Shikano Y. Nishimura Y, Okonogi T, Ikegaya Y, Sasaki T: Vagus nerve spiking activity associated with locomotion and cortical arousal states in a freely moving rat. European Journal of Neuroscience, 2019
- Sasaki T: Non-structured spike sequences of hippocampal neuronal ensembles in awake animals. Neuroscience Research, 2019
- Sasaki T: A physiolomics approach to reveal systemic organ dynamics in a rodent. Biological Pharmaceutical Bulletins, 2019
- Sasaki T, Piatti VC, Hwaun E, Ahmadi S, Lisman JE, Leutgeb S, Leutgeb JK: Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nature Neuroscience, 21, 258-269, 2018
- Sasaki T, Leutgeb S, Leutgeb JK: Spatial and memory circuits in the medial entorhinal cortex. Current Opinion in Neurobiology, 32, 16-23, 2015
- Sasaki T, Matsuki N, Ikegaya Y: Targeted axon-attached recording with fluorescent patch-clamp pipettes in brain slices. Nature Protocols, 7, 1228-1234, 2012
- Sasaki T, Beppu K, Tanaka KF, Fukazawa Y, Shigemoto R, Matsui K: Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proceedings of the National Academy of Sciences of the United States of America, 109, 20720-20725, 2012
- Sasaki T, Matsuki N, Ikegaya Y: Action potential modulation during axonal conduction. Science, 331, 599-601, 2011
Close
Whole-brain analysis of neuronal mechanisms underlying individual differences of stress resilience

-
Project leader
Laboratory of Molecular Neuropharmacology, Graduate School of Pharmaceutical Sciences, Osaka University
Visiting Scholar Kaoru Seiriki
Further information
Message from Project leader
Chronic stress exposure is one of the risk factor for depression; however, vulnerability to stress differs among individuals and its neuronal mechanisms are less understood. Although the neuronal phenotypes and mechanisms underlying stress-resiliency have been identified using animal models after chronic stress exposure, it is unknown whether or how the potential difference of brain function before stress exposure contributes to acquisition of stress-resiliency. The goal of this study is to develop an imaging and analysis method to investigate correlation relationships between individual differences of neuronal activity before stress exposure and depression-like behavior after chronic stress exposure. To achieve this, we employ our whole-brain microscopic imaging technology: FAST (block-FAce Serial microscopy Tomography) to obtain the whole-brain activation data at single cell level, and explore the brain regions contributing to individual differences of stress resilience.
-
Project leader
Visiting ScholarKaoru SeirikiLaboratory of Molecular Neuropharmacology, Graduate School of Pharmaceutical Sciences, Osaka University
Research Area:
pharmacology, neuroscienceE-mail:
seiriki*phs.osaka-u.ac.jp
(Please convert "*" into "@".)
Selected Publication
- Seiriki K, Kasai A, Nakazawa T, Niu M, Naka Y, Tanuma M, Igarashi H, Yamaura K, Hayata-Takano A, Ago Y, Hashimoto H. Whole-brain block-face serial microscopy tomography at subcellular resolution using FAST. Nat Protocols, Epub ahead of print, 2019
- Seiriki K, Kasai A, Hashimoto T, Schulze W, Niu M, Yamaguchi S, Nakazawa T, Inoue KI, Uezono S, Takada M, Naka Y, Igarashi H, Tanuma M, Waschek JA, Ago Y, Tanaka KF, Hayata-Takano A, Nagayasu K, Shintani N, Hashimoto R, Kunii Y, Hino M, Matsumoto J, Yabe H, Nagai T, Fujita K, Matsuda T, Takuma K, Baba A, Hashimoto H. High-Speed and Scalable Whole-Brain Imaging in Rodents and Primates. Neuron 94(6):1085-1100.e6. 2017
- Seiriki K, Kasai A, Kuwaki T, Nakazawa T, Yamaguchi S, Hashimoto H. Critical involvement of the orbitofrontal cortex in hyperlocomotion induced by NMDA receptor blockade in mice. Biochem Biophys Res Commun 480(4):558-63. 2016
Close
