Activities
- Home
- Activities
- Release
- MeCP2 controls neural stem cell fate specificationthrough miR-199a-mediated inhibition of BMP-Smadsignaling
May 26, 2021
MeCP2 controls neural stem cell fate specificationthrough miR-199a-mediated inhibition of BMP-Smadsignaling
Published in Cell Reports
The research group led by Drs. Hideyuki Nakashima, Takuya Imamura and Kinichi Nakashima from the Graduate School of Medical Sciences, Kyushu University revealed that the MeCP2-downstream miR-199a regulates Neural Stem/Precursor Cell (NS/PC) differentiation.
Rett syndrome (RTT) is a severe neurological disorder, with impaired brain development caused by mutationsinMECP2; however, the underlying mechanism remains elusive. We know from previous work that MeCP2facilitates the processing of a specific microRNA, miR-199a, by associating with the Drosha complex to regu-late neuronal functions. Here, we show that the MeCP2/miR-199a axis regulates NS/PC differentiation. A shift occurs from neuronal to astrocytic differentiation of MeCP2- and miR-199a-deficient NS/PCs due to the upregulation of a miR-199a target, Smad1, a downstream transcription factorof bone morphogenetic protein (BMP) signaling. Moreover, miR-199a expression and treatment with BMP in-hibitors rectify the differentiation of RTT patient-derived NS/PCs and development of brain organoids,respectively, suggesting that facilitation of BMP signaling accounts for the impaired RTT brain development.Our study illuminates the molecular pathology of RTT and reveals the MeCP2/miR-199a/Smad1 axis as a po-tential therapeutic target for RTT.
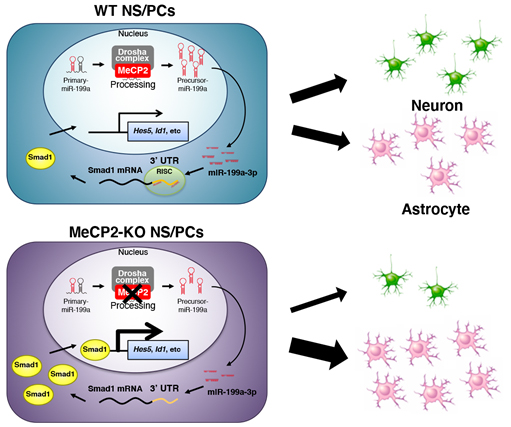
Figure. A model of NS/PC differentiation regulated by the MeCP2/miR-199a/Smad1 signaling cascade. In WT NS/PCs, the MeCP2-Drosha complex facilitates processing of the primary form of miR-199a to its precursor form. The mature miR-199a-3p targets Smad1 mRNA, leading to a reduction of Smad1 protein. Consequently, BMP signal activity is downregulated, while NS/PC differentiation remains normal. In MeCP2-KO NS/PCs, processing of pri-miR-199a to pre-miR-199a is impaired by MeCP2 deficiency, resulting in a reduced level of mature miR-199a. Decreased miR-199a cannot effectively suppress the expression of Smad1, so that the expression of factors downstream of BMP signaling is upregulated, which inhibits neuronal differentiation and promotes astrocytic differentiation of NS/PCs.
Press release
http://www.med.kyushu-u.ac.jp/app/modules/eninfo/detail.php?i=93&c=2
Cell Reports
https://www.cell.com/cell-reports/fulltext/S2211-1247(21)00463-0
